SVET/Vibrating Probe – M470.
A non-intrusive scanning probe electrochemistry technique used to map local electrochemical events in real time.
Scanning vibrating probe, or SVET, is often used by researchers to investigate corrosion processes in situ, in real time.
Scanning Vibrating Electrode Technique measurements are performed on BioLogic’s M470 using the SVP470 option.
Scanning Vibrating Electrode Technique (SVET), also known as Scanning Vibrating Probe (SVP), and Vibrating Probe in the field of biology, is a non-intrusive scanning probe electrochemistry technique. SVET is used to measure the local electric field of a sample in solution, ultimately allowing the current density of a sample to be mapped. SVET achieves a high degree of electrical sensitivity and stability, in particular compared to predecessor techniques, through the vibration of the probe perpendicular to the sample. This sensitivity and stability make SVET well suited to mapping electrochemical events in real-time.
With SVET, events occurring in dynamic samples, can be followed in real time: a factor that is highly advantageous for researchers wishing to follow electrochemical processes as they occur. This characteristic has led to SVET finding widespread use in corrosion and coatings, to investigate, for example, corrosion propagation, coating efficiency, self-healing coatings and more. In the field of biology, for which SVET was introduced, its ability to measure minute extracellular currents has seen it used to investigate biological processes such as growth and healing. Interest in the use of SVET to measure battery materials has been growing.
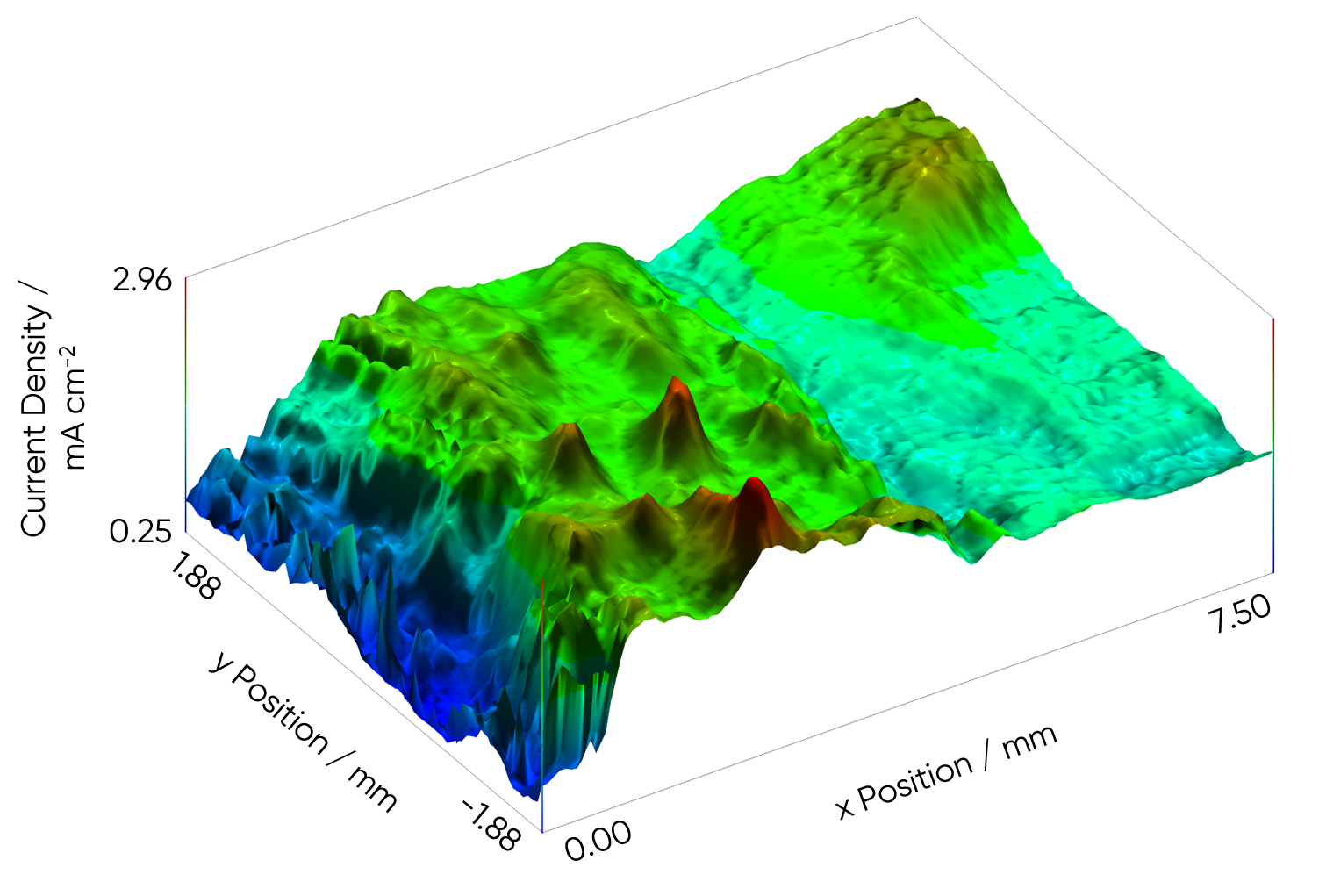
SVET measurement of steel weld measured at Open Circuit Potential (OCP) in
5 mM NaCl with height tracking, using topography measured by OSP470.
Overview: Measure the local current distribution of a sample in electrolyte
- Study dynamic samples in situ
- Visualize anodic and cathodic regions
- Suitable for naturally active and biased samples
Real-time measurements of dynamic samples
SVET experiments benefit from the ability to implement a sweep scan mode, meaning the probe does not need to pause at each measurement point. Repeated upgrades to the BioLogic SVP470 module have ensured that it has excellent electrical sensitivity and system stability ensuring that experiments can be performed quickly and reliably, to clearly visualize anodic and cathodic regions of a sample. SVET measurements with the SVP470, therefore, offer clear advantages for the measurement of dynamic samples in real time, as the electrochemical processes occur in situ and in vivo. It is even possible to sequence multiple SVET scans to automatically follow these processes as they develop, using the M470’s proprietary software.
Locally map the current density of samples in situ
SVET is well suited to in situ studies, due to the measurement of the active, or biased sample in solution. This is key to the experiment with the iR drop in the solution above the sample exploited to allow the measurement of micro-galvanic potentials. While it is common for these potentials to be plotted as is, they can easily be converted to a map of the local current density of the sample, through the calibration of the SVET probe.
Auto-tune capability for faster experimental setup
SVET depends on the vibration of the probe perpendicular to the sample to produce a sinusoidal current signal. This AC current is converted to a DC current by the application of a demodulation signal by the lock-in amplifier of the M470. The demodulation signal applied by the lock-in amplifier must be selected so that its phase maximizes the DC response. To remove the difficulties sometimes associated with determining the best reference phase it is possible to use Auto-Tuning with the SVP470 experiment. Aside from ensuring the maximum DC signal, the use of Auto-Tuning also reduces the setup time of the experiment.
Workstation Software
The Scanning Electrochemical Workstation software provides unique capabilities and interactivity in support of the Model 370 and Model 470 nanometer-resolution scanning probe microscopes. This highly ergonomic software has been designed to facilitate and improve the user experience and render work flows more efficient:
- Improved data analysis, manipulation and interactivity
- Automatic measurement and sequencing functionalities.
Over 40 discrete experiments provided throughout, each with their own individual variations
Specifications
| Scanning Stage | |
| Scan Range (x,y,z) | 110 mm x 110 mm x 110 mm |
| Minimal Step Size (x,y,z) | 20 nm |
| Positioning | Closed loop positioning, linear, zero hysteresis encoder with direct real-time readout of displacement in x, y, z |
| Linear position encoder resolution | 20 nm |
| Max Scan Speed | 10 mm/s |
| Measurement Resolution | 32-bit decoder @ up to 40 MHz |
| Dimensions | 500 mm (H) x 400 mm (W) x 675 mm (D) |
| SVP Electronics | |
| Signal Chain | Phase-sensitive detection using microprocessor-controlled lock-in amplifier with a digital dual-phase oscillator and differential electrometer input. |
| Lock-In Amplifier | Software controllable gain range (1 – 105) |
| Output time constant: 0.01, 0.1, 1, 10 s | |
| Differential Electrometer | 1015 Ω input impedance |
| Decade gain ranges: 0 to 80 dB | |
| Common mode range: ±12 V | |
| Vibration Actuator | One dimensional low voltage piezo-electric actuator |
| Vibration Amplitude | Software set from 1 – 30 μm perpendicular to sample surface |
| Electrochemical Sensitivity | Better than 5 μA/cm² (using standard PIS test approach) |
| General | |
| Available Experiments | Line Scan, Area Scan |
How do I use the M470?
Connection of 3300 potentiostats to the cell
Connection of SP-300 potentiostats to the cell
Setting up the probe & building the MicroTriCell
Experimental conditions and set-up
Probe characterization
Sample characterization
Approach experiment on resin (insulator)
Approach experiment on Gold (conductor)
Line Scan and Area Scan
Is there any analysis software available for use with the M470?
What application areas can I use the M470 for?
- SECM for bio-sensors
- Application of local electrochemical probes for coatings studies
- Application of local electrochemical probes for corrosion studies
- Application of local electrochemical probes for energy studies
- Application of scanning probe electrochemistry for biological studies
I see you supply 1 µm probes. Can I use these with the M470?
The following application note relates to 1 µm probes
Can I perform constant distance measurements?
The following application notes relate to distance measurements…
AN#1 – Height tracking with the SKP370 or SKP470 module
AN#2 – SECM height relief with OSP: An application in corrosion
AN#3 – SECM height relief with OSP: an application in sensors
AN#6 – Advantages of the intermittent contact SECM: two examples in corrosion
AN#11 – Measurement of a nano-patterned gold sample by ic-/ac-SECM
AN#13 – Investigation of an interdigitated array electrode using ic-SECM
AN#16 – Intermittent Contact (ic) SECM for relief of major topographic features
TN#14 – Height Tracking Inputs for SKP Investigations
Related topics