What is a potentiostat and its use in Science & Industry (Electrochemistry Basics Series)
Latest updated: June 27, 2023The role of potentiostats, the swiss-army knives of electrochemical research, and their value in R&D, quality control, and battery testing amongst other industrial and scientific domains is examined in this article.
Definition of a potentiostat
A potentiostat galvanostat is an electronic device used in electrochemistry that controls the potential difference (voltage) and current. It is a combination of a potentiostat, which controls the voltage across an electrochemical cell, and a galvanostat, which controls the current through the cell. It is commonly used to study and control electrochemical reactions, including those involved in corrosion, electrodeposition, and battery testing.
Behind every good decision in electrochemical research, lies a good potentiostat…
Measurement and analysis tools have long been used in the worlds of industry and research to drive innovation. As well as increase our scientific and technical understanding, such tools play a vital role in quantifying and qualifying phenomena imperceptible to human senses. Potentiostats are a great example of such analytical devices and have supported progress in the field of electrochemistry for more than a century.
Potentiostats (sometimes referred to as electrochemical workstations or potentiostats / galvanostats) are vital measurement and control tools used primarily in electrochemical research, as well as in other industrial fields. In electrochemistry, potentiostats are used both in fundamental and applied research to gain an increased understanding of electrode processes, analytical chemistry, battery research, and corrosion research. Secondary applications include chemical synthesis and biology. You will see a full list of these applications in this article.
Potentiostats have proven to be major tools in the development of secondary batteries through their ability to study electrochemical interfaces. They also enable battery testing through their ability to charge and discharge a battery under pre-defined conditions.
This article will give a broad overview of the role of the potentiostat in research and industry and will describe how they work.
At a glance: The definition of the instrument. What is a potentiostat / galvanostat and what does it do?
A potentiostat / galvanostat is an instrument that manages the application of voltage or current to an electrochemical cell electrode (detailed description below). The potentiostat / galvanostat is the main tool used in electrochemical and electroanalytical experiments.
Overview: function modes
Potentiostatic vs Galvanostatic modes
Potentiostats / galvanostats have two main function modes depending on the electric quantity being controlled – the potential or the current. These are called the potentiostatic and galvanostatic modes (see Figures 1 & 2 below). When the potentiostat/galvanostat is used in potentiostatic mode, it can be referred to as a potentiostat.
In potentiostatic mode, the potentiostat applies and controls the potential, and measures the current flowing through the electrochemical system (see Figure 1 below).
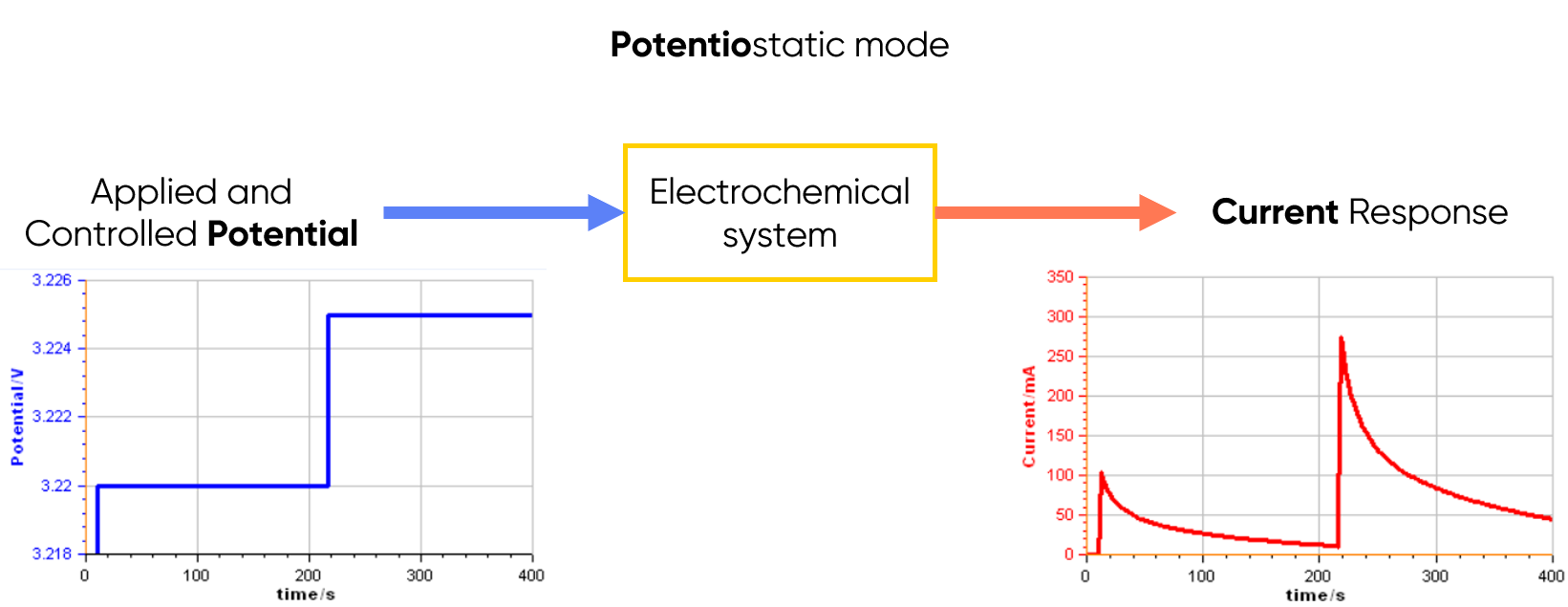
Figure 1: Principle of the potentiostatic mode.
At a glance: What is Potentiostatic mode?
The potentiostatic mode measures and controls the voltage difference between a working electrode and a reference electrode, which has a constant potential. This, perhaps the most commonly used mode of an electrochemical workstation, measures the current flow between the working electrode and counter electrode (that completes the cell circuit).
It is common to apply a “voltage ramp” where voltage is applied incrementally (a potentiodynamic technique) the most popular being Cyclic Voltammetry (CV). Cyclic Voltammetry is a fast and easy technique to obtain the complete behaviour of an electrochemical system (see Cyclic Voltammetry: how to obtain great results with your potentiostat).
The techniques most commonly used in corrosion and analytical electrochemistry are based around the potentiostatic mode.
In galvanostatic mode, the electrochemical instrument performs the measurements for potential variations while the current is applied and controlled (see Figure 2 below).
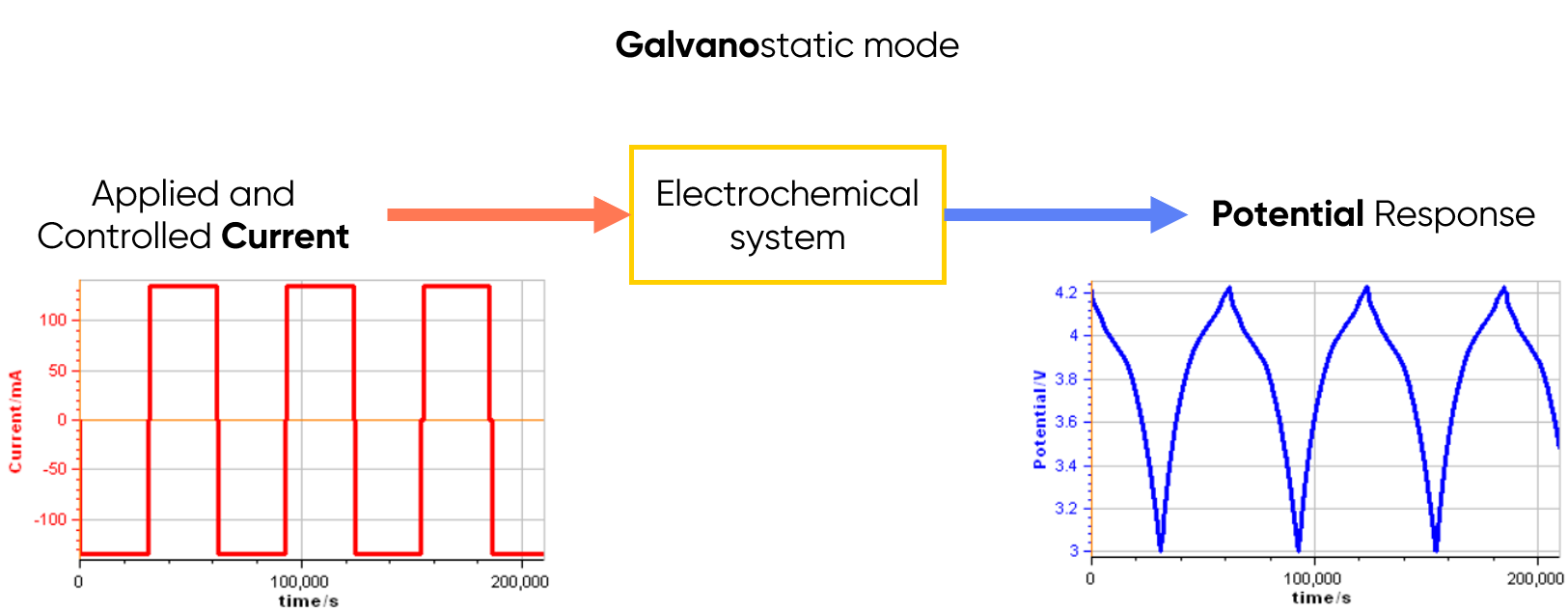
Figure 2: Principle of the galvanostatic mode.
At a glance: What is Galvanostatic mode?
Galvanostatic mode is based on the control of current flowing through the system. When the device is used for these measurements it is called a galvanostat. The most common application for galvanostatic mode is research into batteries.
Lots of modern electrochemical techniques are based on a succession of sequences using alternatively potentiostatic and galvanostatic modes. The ability to switch from one mode to another is a relatively recent development in potentiostat design but one that is used extensively in battery testing where there is a need to switch in rapid succession from potentio to galvano mode. Such functionality requires high-end specifications such as those available on BioLogic potentiostats.
Other function modes
Potentiostats also have specific control modes: the Open Circuit Voltage (OCV), the Zero Resistance Ammeter (ZRA) and by the Electrochemical Impedance Spectroscopy (EIS). Each of these control modes has a specific purpose.
OCV control mode enables the acquisition of voltage data when the cell is in a resting state i.e. when the potentiostat is not applying any current or voltage to the working electrode of the cell. This control mode is commonly used for for equilibration of the electrochemical cell.
EIS designates a sinusoidal control mode. The potentiostat applies a sinusoidal input signal (current or voltage) and measures the response of the system. This control mode is widely used in electrochemical and corrosion systems because it provides detailed information about reaction kinetics, corrosion rates, and mass transfer parameters, amongst others. For more information about electrochemical impedance spectroscopy (EIS), please visit the Learning Center article “What is EIS”.
The ZRA control mode allows the determination of electrochemical current noise by applying a voltage equal to 0 V between the working electrode and the counter electrode (see description of electrodes) and measuring the current flowing between the two identical samples. More information about the electrochemical noise measurements can be found in Application Note #39-1.#39-2, #39-3
A seamless link between the potentiostat interface (software) and the potentiostat (hardware) makes it easy for the user to manage each mode.
In the next part of the document, we will look at exactly how the potentiostat works by examining the key components and the potentiostat’s architecture.
How do potentiostats / galvanostats work?
Controlling the potential: the three-electrode setup
The potentiostat is an instrument dedicated to the study of electrochemical processes. The control of the interfacial working electrode potential is crucial to guarantee that current is measured at a constant potential. A three-electrode setup makes this possible.
These three electrodes are known as the working electrode, the reference electrode, and the counter-electrode (also called the auxiliary electrode).
 | The working electrode (WE): The reaction of interest occurs at the interface of the working electrode. The reference electrode (RE): The potential of the reference electrode is well-known and stable. It is the point of reference of the system for potential control and measurement. The current flow through this electrode is kept close to zero. The counter-electrode (CE): The current flows between the working electrode and the counter-electrode. The counter-electrode has no role in electrochemical reactions except for specific situations: battery cell, galvanic corrosion, electrochemical noise measurements. |
Figure 3: Example of a three-electrode setup.
Key potentiostat components: reference electrode and control amplifier
To guarantee that currents flows as a result of potential variations at the working electrode interface, the potential of the reference electrode must remain stable and correspond to its theoretical value. It must be properly maintained. (see Checking and Validating reference electrodes).
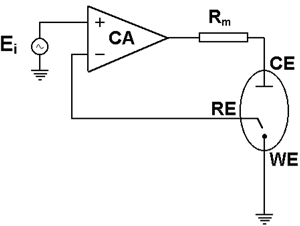
The control amplifier (CA) is a key electronic component in the potentiostat. It is used to keep the voltage between the reference electrode and the working electrode as close as possible to the voltage of the input source $E_{\mathrm{i}}$. The potentiostat circuit diagram in Figure 4 shows the position of the control amplifier in a simplified design of a modern potentiostat [1].
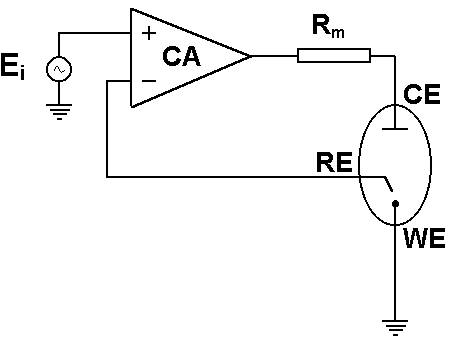
Figure 4: Circuit diagram of basic potentiostat design [1].
The potential measured at the reference electrode is fed back to the negative input of the control amplifier. This creates a loop called the “negative feedback loop”, which allows the control amplifier to adapt its output and maintain a potential difference corresponding to $E_{\mathrm{i}}$. This allows the potentiostat to precisely control the voltage signal and reach the right setpoint value. For more information, see the BioLogic Application Note #04: “The mystery of potentiostat stability explained”.
In the next part of this article, we will describe the technological evolution of potentiostats over time, from their initial invention to the very latest advances in potentiostat development.
Looking back: The history of potentiostats
The first potentiostatic method was used by F. G. Cottrell in 1903 who worked on the verification of mass transfer equations. His experiment consisted of an electrochemical cell connected to a battery in series with a galvanometer for the measurement of a current. However, at this point in time, with such a simple potentiostat, it was not possible to ascertain the potential difference at the interface of the working electrode in the circuit.
Hickling, an electrochemist at the University of Leicester developed, in 1942, the principle of the modern three-electrode potentiostat, that we now know today. Hickling assumed that electrolytic processes are generally governed by the electrode potential, which, until then, was only indirectly controllable by modifying temperature, current density, or electrode materials [2]. Hickling added a third electrode to the system and developed a means of automatically controlling the potential. The general principle behind this design was to compare the potential difference between the working electrode and the reference electrode using voltage derived from a potentiometer [3]. This principle used in the Hickling potentiostat is based on negative feedback technology (see above).
The Hickling potentiostat was the first potentiostat to use a negative feedback circuit for the measurement and control of the electrodes’ voltage. In 1956, the electrochemist Prazak used the word potentiostat to describe Hickling’s electrochemical instrument.
Another important contributor to potentiostat development was Hans Wenking who solved many problems for electrochemists in the late 50s and 60s by contributing to the design of electronics still used in the modern electrochemical workstations of today [4].
Since 1971, negative feedback has been achieved using an electronic component, called a control amplifier [5]. To find out more about negative feedback, please visit the BioLogic Learning Center article “Get more from your potentiostat. Understanding bandwidth & its effect on measurements”.
From the 70’s onwards, electrochemical impedance spectroscopy (EIS) has become a major tool in the characterization of electrochemical systems. Since then, potentiostat technology has constantly advanced with huge improvements made in terms of functionality, performance, and precision. One of the most challenging issues when designing a potentiostat relates to the ohmic drop phenomenon (see article “Ohmic drop correction: a means of improving measurement accuracy”). These versatile electrochemical instruments have evolved from simple potentiostats to potentiostats / galvanostats, with two main modes of operation (see potentiostatic and galvanostatic mode definitions above).
Furthermore, they have become far easier to use and increasingly more powerful. The first example of a multichannel computer-controlled potentiostat could be seen in 1991 with the launch of the MacPile. This potentiostat was invented by two French researchers, Yves Chabre and Christian Mouget, and commercialized by BioLogic. The MacPile can be considered as the precursor to the vast array of potentiostat / galvanostats now available on the market.

Figure 5: The world’s first multichannel computer-controlled
potentiostat –The Mac Pile, launched in 1991.
The principle of negative feedback inspired by A. Hickling remains to this day the cornerstone of potentiostat design. The following paragraph explains how modern potentiostats work.
Applications: How are potentiostats used in academic research and in industry?
In which industrial and scientific applications are potentiostats used?
Potentiostat / Galvanostats appear to be an essential analysis tool in scientific as well as in industrial fields. They are helpful in a wide range of applications and domains such as energy storage and conversion, electrochemistry, materials science, and life sciences, to mention but a few. Figure 5 below gives an overview, and for more information, you can learn about potentiostat use by application in the BioLogic Learning Center.
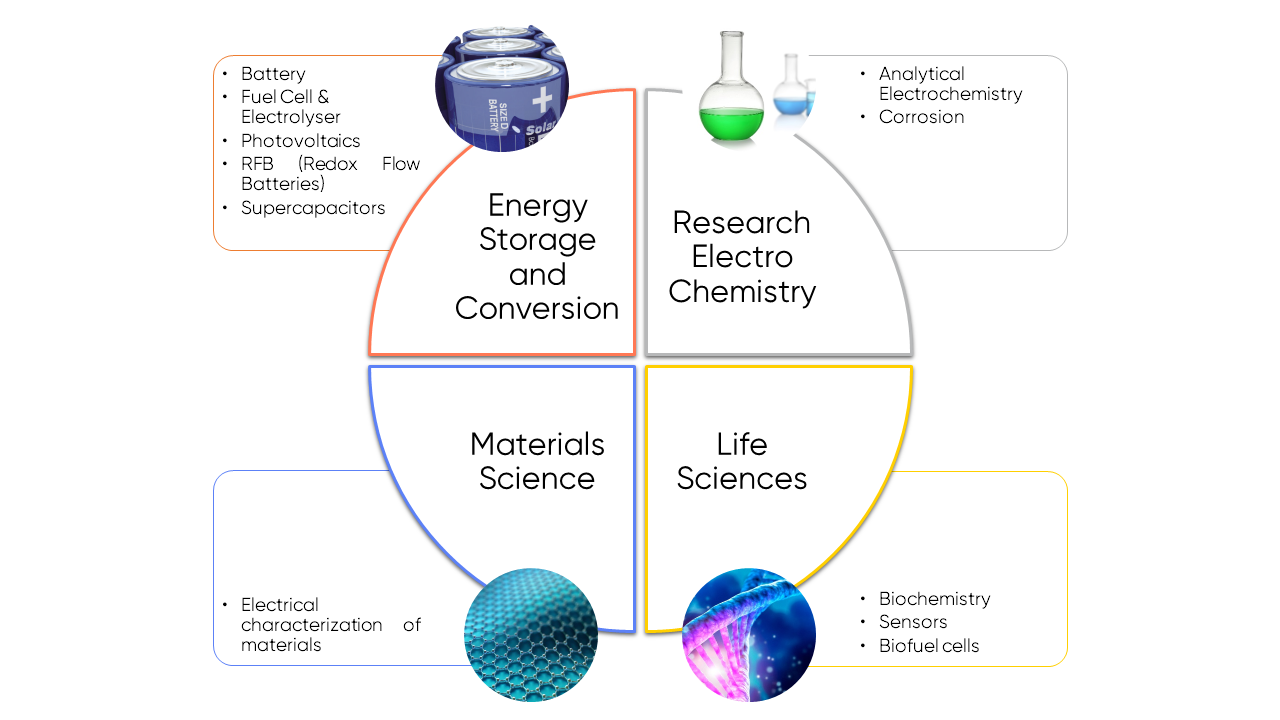
Figure 6: Potentiostats are present in a wide range of industrial and scientific applications
Focus on electrochemistry and energy storage: two of the major techno-societal challenges where potentiostats are advancing research and industry
Potentiostats and sensors
In most electrochemistry applications, potentiostat / galvanostats contribute significantly towards increased understanding, research, and the development of electrochemical systems.
Because potentiostats allow the observation of redox reactions, the potentiostat is the traditional tool of choice for laboratories specializing in analytical electrochemistry. For example, VMP-300 potentiostats can be used to characterize species or electrochemical processes using the cyclic voltammetry technique of BioLogic software EC-Lab®.
Similarly, cyclic voltammetry and other electrochemical measurements (differential pulse voltammetry and electrochemical impedance spectroscopy) have been used with single-channel potentiostats such as the BioLogic SP-50 to study the electroanalytical properties of a fabric immunosensor. These wearable sensors monitor the concentration of cortisol in human sweat non-invasively and in real-time. The cortisol level in the human body is a valuable source of information since a high concentration of cortisol can warn of the presence of a tumor [6].
Potentiostats and Corrosion
Corrosion is also a major focus within electrochemistry and an area where potentiostats play a vital role. The global cost of corrosion is estimated at $2.5 trillion [7] with corrosion control having the potential to save 375-875 billion dollars annually (a staggering 3.4% of global GDP in 2013). See our Learning Centre article Coatings, Corrosion and Scanning Probe Electrochemistry. Corrosion can have a devastating effect on infrastructure, production/manufacturing, and transportation amongst many other activities which are dependent on the integrity of the metal used. Electrochemical workstations (potentiostats) are used in general electrochemistry to help better understand the fundamental electrochemical processes that lead to the degradation of metal in reaction with oxidants such as oxygen or sulfates.
Potentiostats are also used in materials science to help develop coatings and prevent corrosion. They are very useful for such analyses because they allow the study of corrosion rates. For example, material properties and structures on the corrosion properties of the material can be studied [8]. As well as potentiostats, scanning probe workstations are used to better understand local electrochemical processes occurring during corrosion. Scanning probe workstations give a “micro” view of corrosion, as opposed to the “macro” view obtained with potentiostats (for more information, please see the article “Coatings, Corrosion and Scanning Probe Electrochemistry”). An example of scanning probe workstations use in corrosion studies is the analysis of the impedance homogeneity distribution of a material thanks to LEIS (Localized Electrochemical Impedance Spectroscopy) technique. This scanning probe electrochemistry technique provides detailed information on the corrosion resistance of the system [9].
Energy storage and batteries: Potentiostat use at every level of the battery value chain
The advances made in battery research in the last decade are nothing short of staggering. In 2010 the lithium-ion battery came to the fore with the exponential growth of the telecommunications industry and market penetration of the mobile phone. A period of rapid technological development means that we now see lithium-ion batteries powering pretty much everything we see in our modern world – even cars. Climate change means that the sword of Damocles hangs over much of the developed world with the demise of fossil fuels and a vital need to find replacement energy sources. To this end, energy storage is critical to the successful development of alternative energy strategies – and at this moment in time, batteries look likely to be the most effective technology capable of harnessing the power of the sun, sea, and wind. It is therefore not difficult to understand why the battery market is so important. Indeed, the global battery market is an area of high growth and is expected to reach $279.7 Billion by 2027 [10].
Potentiostats are so important in research because they allow the user to characterize each part of a battery cell, but also stress the cell in its final design in order to characterize its performance, through the repeated process of charge/discharge, known as battery cycling. As battery performance becomes increasingly important, potentiostat quality and the system’s capability to lead to appropriate data of interest also becomes critical.
The role of the battery cycler
Potentiostats have found favor in a variety of industrial fields, mainly thanks to the proliferation of batteries across a wide variety of fields, but especially automotive and telecommunication industries. With the growth of the secondary (rechargeable) battery market, a need for a new electrochemical measurement instrument has arisen – the battery cycler. Battery cyclers share many of the characteristics of the potentiostat, but this electrochemical measurement tool is used to answer two primary needs. Manufacturers use battery cyclers to control the quality of the batteries they produce. Major industrial organizations buy these batteries and then integrate them into their products. Battery cyclers are then used by industry to select and qualify batteries. Often, these tests involve battery cycling in order to quantify their capacity.
During its life, the battery can be associated with a battery management system (BMS) which adapts battery use in relation to its State-of-Charge or State-of-Health (see our Learning Center article: “Battery states: State of Charge (SoC), State of Health (SoH)”). After its initial use, the internal resistance of the battery (whose evolution is related to the aging process) can be quantified using Electrochemical Impedance Spectroscopy (EIS) in order to determine the next step for managing battery life (“second life” is a term widely used for the upcycling and recycling of batteries).
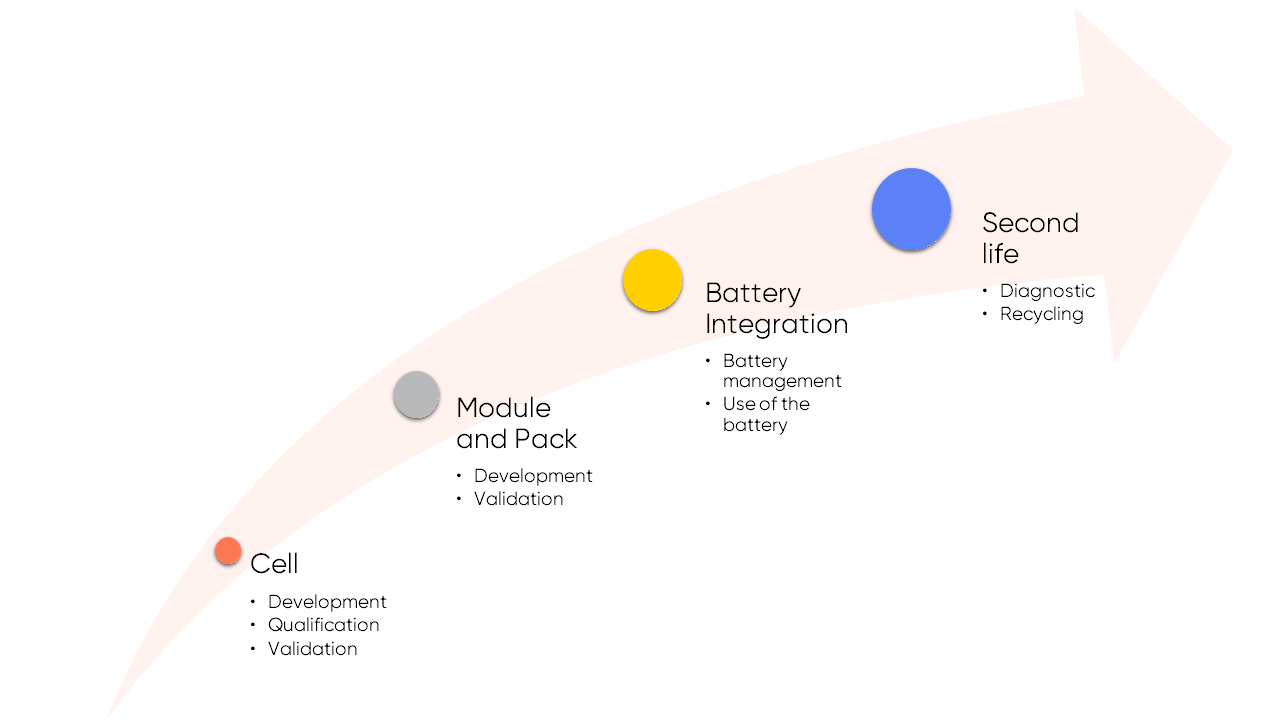
Figure 7: The technology value chain for batteries.
Looking forward: The future of potentiostats…
In this article, we have cited but a few examples of the many applications and opportunities offered by potentiostat / galvanostats. This electrochemical multi-tool plays a vital role in laboratories, research institutes, and R&D centers across the globe. In all applications, there is an increasing need for higher-accuracy, higher-precision, higher-performance instruments that will drive potentiostat research and improved potentiostat specifications. Sensor research is another application demanding ever-more performant analytic tools. And the colossal damage caused by corrosion (especially seawater) means that the next generation of potentiostats will continue to play a vital role in minimizing damage to infrastructure through fundamental research and research into coatings and other technologies.
For energy storage, new battery chemistries are constantly evolving. New generation lithium-ion offers the highest level of energy density currently available. Solid-state versions of the Lithium-sulfur chemistry are expected to offer excellent potential for space and aviation sectors as well as the automotive industry. Finally, the development of solid-state batteries will necessitate ever more powerful potentiostats with increased functionality and performance. The potentiostat has already found its place in fundamental and applied research, R&D, and industrial applications. But we have not yet started to see the full value of these extraordinary measurement tools.
To know more about BioLogic potentiostat galvanostats, have a look at the following video ; and for a more detailed overview of essential and premium potentiostat ranges, please click here.
REFERENCES
- Application Note #04 “The mystery of potentiostat stability explained”.
- A.Hickling, Studies in electrode polarisation, Part IV, (1942).
- A.Hickling, Electrochimica Acta, Vol 5, (1961) 161-168.
- R.Dölling, Materials and Corrosion, 49, (1998) 535-538.
- B. Petrescu, Système électroanalytique flexible contrôlé par ordinateur, Thèse de l’Institut National Polytechnique de Grenoble et de l’Université “Politehnica” de Bucarest, 2002.
- S. Madhu, A. J. Anthuuvan, S. Ramasamy, P. Manickam, S. Bhansali, P. Nagamony, V. Chinnuswamy, ACS Applied Electronic Materials, 2, (2020) 499-509.
- International measures of prevention, application, and economics of corrosion technologies study, Nace International report
- H-R. Erfanian-Nazif’Toosi, H. F. Lopez: Journal of Materials and Applications, 9(1), (2020) 1-8.
- T. Liu, Y. Wang, S. Pan, Q. Zhao, C. Zhang, S. Gao, Z. Guo, N. Guo, W. Sand, X. Chang, L. Dong, Y. Yin, Corrosion Science, 149, (2019) 153-163.
- Global Battery Market Report 2020-2027, ResearchAndMarkets.Com
GLOSSARY
| Term | Definition |
| Battery cycling | Testing process technique of batteries based on repeated and successive charge and discharge phases. |
| Control amplifier | Main active electronic device & part of the analog control loop of a potentiostat, delivering power to an electrochemical cell . |
| Current | Physical quantity describing the flow of charged particles (electrons, ions) in a conductor (SI unit: A) |
| Counter electrode | Auxiliary electrode allowing current to flow through the cell. |
| Electrode potential (Voltage) | The quantity which describes the potential difference between both sides of the electrode interface (SI unit: V). |
| Internal resistance | Generic term that does not designate a specific resistance in the battery. It is a loose characteristic of the battery. |
| Negative feedback | The loop operated by the control amplifier. |
| Potentiostat / Galvanostat | Electronic device capable of applying a voltage and measuring the current response (or vice versa) of an electrochemical interface. |
| Reference electrode | Electrode used to measure the potential difference of an electrochemical interface. Its own potential is stable because it is not traversed by a current. |
| Working electrode | An electrode on which the reaction of interest occurs. |
How to use your potentiostat? Visit BioLogic's Learning Center
Related products