Why use Electrochemical Impedance Spectroscopy (EIS) for battery research?
Latest updated: June 27, 2023Introduction
Impedance measurements can be used to monitor and control the degradation of the performance of a battery during cycling. Electrochemical Impedance Spectroscopy (EIS) measurements are usually performed at higher frequencies to determine the internal resistance of an element. The impedance related to electron transfer at the electrode interface through the solid electrolyte interphase layer (SEI) can also be very important in battery optimization. In the case of batteries involving intercalation of species, at lower frequencies, impedance measurements can also prove to be useful, as in certain conditions it is possible to extract the diffusion coefficient of the intercalated species. This quantity is also of great importance in the processes for the optimization of battery materials and also monitoring.
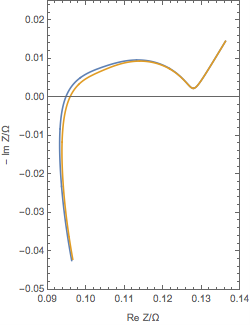
The figure below shows a typical Nyquist plot of the impedance of the negative electrode of an LiB.
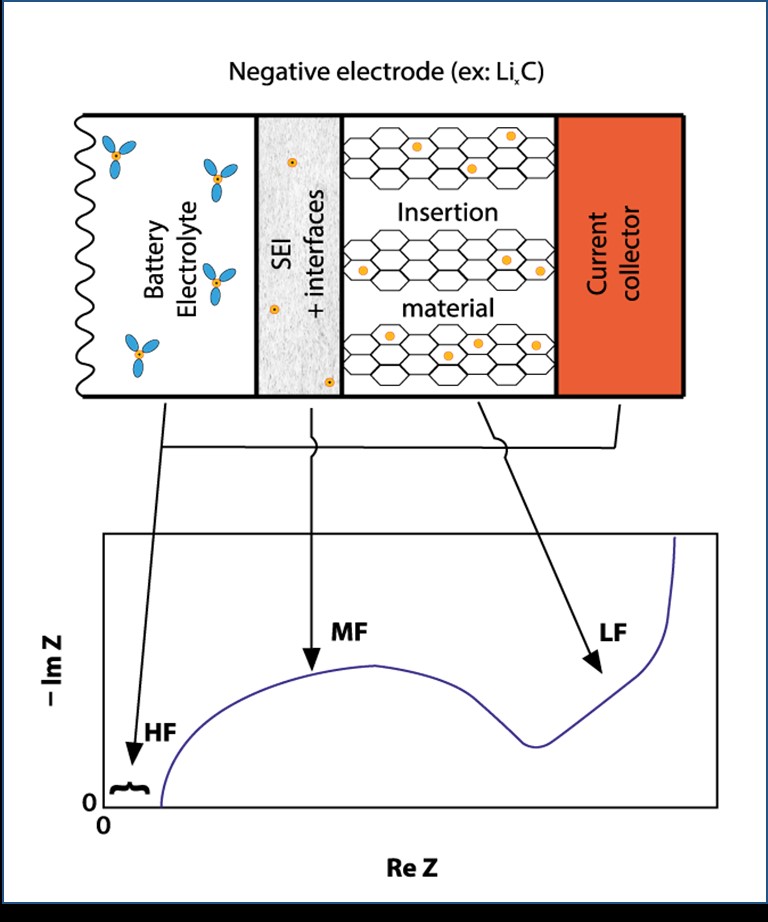
Each component of the half-cell can be characterized. At high frequencies, one can measure the ohmic resistance of the electrolyte. At middle frequencies, one can obtain information concerning SEI capacitance and electron transfer rate. At lower frequencies, one may obtain information about the diffusion processes of species in the insertion material.
EIS capability is available in BioLogic potentiostats / galvanostats as well as battery cyclers. The impedance fitting tool within EC-Lab®, ZFit, make the analysis of Nyquist plots far easier.
For more information please see the following Application Notes:
Related products
Related accessories