SECM101: Introduction to the redox mediator
Latest updated: August 9, 2023Local electrochemistry: The importance of the redox mediator
When designing a Direct Current-Scanning Electrochemical Microscopy (dc-SECM) experiment the redox mediator, an electrochemically active molecule critical to the experiment must be considered. The options for redox mediators can, at times, seem limitless, causing uncertainty during experiment planning. When selecting the redox mediator researchers should consider the electrochemical interaction of interest, how a given mediator interacts with the sample, the redox potential of the mediator, and more.
The role of the redox mediator
To understand why the redox mediator is key to the dc-SECM measurement it is important to understand how the dc-SECM measurement works. In dc-SECM the probe is biased to interact with the redox mediator, which diffuses between the sample and the probe. When the probe is very near an insulating region the diffusion of the redox mediator to the probe is blocked, causing the biased probe to measure a lower current than in bulk electrolyte, Fig. 1. On the other hand, when the probe is very near a conductive (active) region the redox mediator will be re-generated (or generated) by the sample as it is consumed by the biased probe causing a higher current than in bulk to be measured, Fig. 2. It is this interaction of the redox mediator with the probe which is of interest in the dc-SECM experiment, and therefore the redox mediator is an important component to consider when designing any dc-SECM experiment.
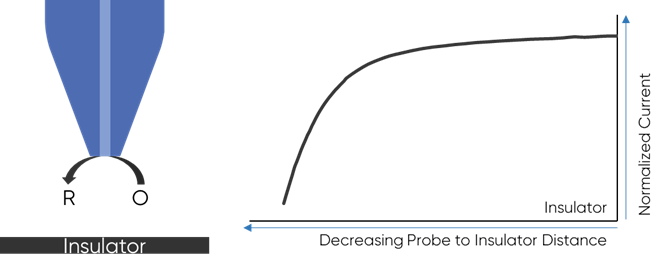
Figure 1: When the probe is very near an insulator the diffusion of the redox mediator
to the probe is blocked resulting in a decrease in SECM signal.
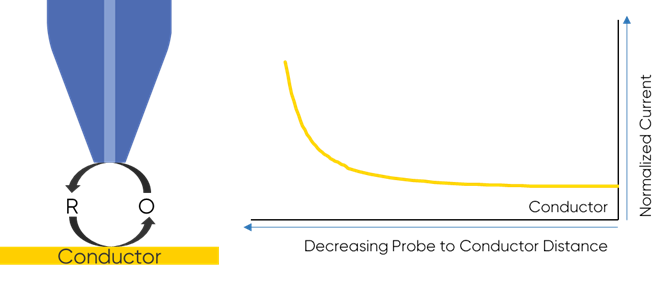
Figure 2: When the probe is very near a conductor the redox mediator is
re-generated by the sample resulting in an increase in SECM signal.
Selection of a redox mediator
There are a number of questions to be answered when selecting a redox mediator for use in a dc-SECM measurement.
Does a redox mediator need to be added to the electrolyte?
Although a redox mediator is necessary to perform a dc-SECM experiment, it is not required that this be artificially added to the measurement electrolyte. There are two classes of redox mediators: indirect and direct mediators [1]. An indirect mediator is one that is added to the measurement electrolyte to allow the sample activity to be probed, as is the case in many feedback mode experiments. Examples of indirect redox mediators include [Fe(CN)6]3-, and I–. On the other hand, a direct redox mediator is one that is naturally present in solution or produced by the probe or sample, as is the case in many generator-collector experiments. Examples of direct mediators include O2, and Fe2+. If there is an appropriate direct redox mediator which can be measured within the conditions of the experiment then the user does not need to add a redox mediator to the electrolyte. This is the case for the measurement shown in Fig. 3, in which the Fe2+ produced by a steel weld in 0.1 M NaOH was measured.
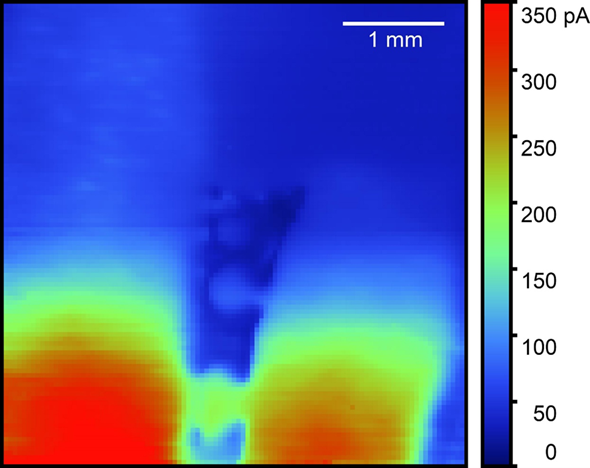
Figure 3: The steel weld was measured in 0.1 M NaOH. The probe was biased at 0.6 V
vs AgAgCl to oxidize Fe2+ to Fe3+, an example of a direct redox mediator.
Is a particular electrochemical interaction of interest?
dc-SECM is a chemically selective technique because the probe can be biased to interact with a specific electrochemically active species, it is therefore at times used to look at specific electrochemical interactions. When it is important to understand how a sample interacts with a given electrochemically active species, this species can be used as the redox mediator in the dc-SECM experiment. This is the case for some catalysis studies in which the redox mediator is selected because the sample is catalytic towards it. An example of this is the high throughput SECM studies of the Oxygen Reduction Reaction (ORR) at thin-film Pt catalysts by Lu et. al., in which the tip produced the O2 redox mediator catalyzed by the thin film sample [2].
Is there a poor interaction between the redox mediator and the sample?
When selecting a redox mediator, it is important to consider how it will interact with the sample. If a mediator is likely to interact poorly with the sample a different mediator should be considered. For example, when measuring living cells some redox mediators may be toxic to the cell. When this is the case the results may not reflect the conditions of interest and can have further unwanted side effects such as biological material coating the probe and reducing the signal. In these cases, the use of a different mediator should be considered.
Is the redox reaction under diffusion control?
dc-SECM measurements are typically performed under diffusion control to obtain the clearest images and to conform to theory. It is important, therefore, to consider whether the redox mediator undergoes a diffusion-controlled redox reaction within the electrochemical window of interest. When a redox reaction is diffusion-controlled it is apparent by a plateau in current in the cyclic voltammetry, as illustrated in Fig. 4.
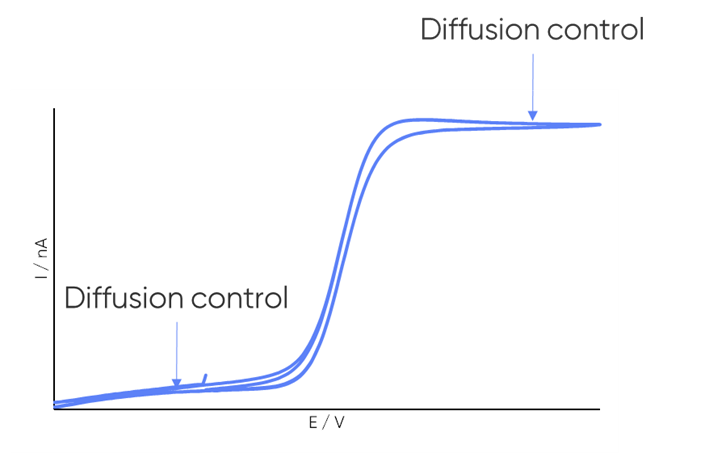
Figure 4: Example cyclic voltammogram annotated to illustrate the diffusion-controlled region.
Is the redox mediator stable under the selected experimental conditions?
It is important that the redox mediator used in a dc-SECM experiment is stable under the required experimental conditions, this includes the electrolyte, atmospheric conditions, temperature, and bias potential selected. If the redox mediator is not stable its decay will result in poor signal strength and quality.
Is the redox potential of the mediator within the electrochemical window of the electrolyte?
When selecting a redox mediator, it is also important to consider its redox potential. To use a given redox mediator its redox potential must be within the electrochemical window of the electrolyte. If this is not the case it may still be possible to use the redox mediator if the probe material is changed.
Options for mediators
There are many possible options for redox mediators in dc-SECM measurements, with extensive lists of these existing in the literature [1, 3, 4]. Some examples are given in Table 1 below.
Table 1: A limited number of examples of redox mediators used in dc-SECM are given.
What if a suitable redox mediator cannot be found?
Even with the wide range of redox mediators which have previously been used in dc-SECM measurements, in some cases, it is not possible to find a suitable redox mediator. This can occur, for example, when the mediator affects the processes under study, as is sometimes the case for corrosion studies, or when it is toxic to a living cell under study. In these cases, the closely related Alternating Current (ac)-SECM technique, which is performed almost exclusively without the use of a redox mediator, should be considered instead.
| Mediator | Redox reaction | E0 (V vs. Ag/AgCl) |
| FcMeOH (Ferrocene Methanol) | [FcMeOH] + e → FcMeOH | 0.303 |
| Fe(CN) 6 (Ferricyanide) | [Fe(CN)6]4 + e→[Fe(CN)6]3+ | 0.294 |
| I (Iodide) | I3- + 2e → 3- | 0.766 |
Conclusion
The redox mediator is a key component of the dc-SECM experiment. While there are a wide variety of mediators available some care is required in selecting the best one for a given experiment. Even when a suitable mediator cannot be found the SECM technique can still be used in the form of ac-SECM.
References
- D. Polcari, P Dauphin-Ducharme, J. Mauzeroll, Chem. Rev. 116 (2016) 13234−13278
- G. Lu, J. S. Cooper, P. J. McGinn, Electrochim. Acta 52 (2007) 5172–5181
- A. J. Bard, in: A. J. bard, M. V. Mirkin (Ed.), Scanning Electrochemical Microscopy Second Edition, CRC Press, Boca Raton, (2012) 1
- N. A. Payne, L. I. Stephens, J. Mauzeroll, CORROSION 73 (2017) 759-780
Related products