ac-SECM101: An Introduction to Alternating Current-Scanning Electrochemical Microscopy
Latest updated: June 2, 2023What is Alternating Current-Scanning Electrochemical Microscopy?
Alternating Current-Scanning Electrochemical Microscopy (ac-SECM) is a form of Scanning Electrochemical Microscopy in which a sinusoidal bias is applied to the SECM probe to indirectly measure the impedance response of the sample under study, it is related to the bulk Electrochemical Impedance Spectroscopy (EIS) technique. ac-SECM can be performed with raster scanning of the probe to allow maps of local impedance at a single ac frequency to be collected. The probe can also be held stationary to allow the measurement of individual EIS spectra at a given point on the sample.
The application of an alternating current to the SECM probe was first introduced in 1993 as a means of determining probe to sample distance without the need for a redox mediator [1]. In 2002 it was demonstrated that the use of an alternating current could also indirectly allow the measurement of the local electrochemical activity of a sample [2], with the advantage over dc-SECM that it did not require the use of a redox mediator to do so.
This article will only consider ac-SECM. For information on dc-SECM please see our previous Learning Center article: SECM101: An Introduction to Scanning Electrochemical Microscopy. Further information on the wide array of Scanning Electrochemical Microscopy types can also be found in the Learning Center.
How does Alternating Current-Scanning Electrochemical Microscopy work?
The principles of Alternating Current-Scanning Electrochemical Microscopy are very similar to those of traditional dc-SECM. In ac-SECM, a sinusoidal bias is applied to the UltraMicroElectrode (UME) probe, which, when in close proximity to the sample allows the sample impedance to be measured.
The impedance measured by the probe is dependent on the electric nature of the sample under investigation. When the probe is in close proximity to the sample surface only a thin electrolyte film exists. When the probe is over an insulating region the field lines from the probe to counter electrode are blocked, resulting in an increase in impedance. For all measurement frequencies, and electrolyte conductivities when the SECM probe is approached to an insulating sample the impedance will increase with decreasing probe to sample distance, Fig. 1. On the other hand, if the probe is over a conducting region of the sample the field lines can also pass through the conducting region of the sample to the counter electrode.
This short circuit results in a lower impedance being measured over these conducting regions, compared to the insulating regions. The electric nature of the sample is not the only factor to affect the impedance feedback as the probe is approached to the sample surface. When the probe is approached to a conducting sample this is also dependent on both the conductivity of the measurement electrolyte and the frequency of the applied ac bias. When a conducting sample is measured in a high conductivity electrolyte, or a low measurement frequency is used, the lowest resistance path to the counter electrode is through the electrolyte, hence the situation is the same as for an insulating sample. Fig. 2. On the other hand, if a conducting sample is measured in a low conductivity electrolyte, or a high measurement frequency is used, the path of lowest resistance for the charge transfer is through the sample (as the double layer capacitance can be shorted).
Decreasing the probe to sample distance reduces the length of the conductor formed by the electrolyte “tube” between the probe and the sample, hence decreasing the resistance between the tip and the substrate. In this case, decreasing probe to sample distance results in a decrease in the impedance measured, Fig. 3. It should be noted that for a conductive sample, the insulating/conducting transition frequency decreases as the conductivity of the electrolyte also decreases.
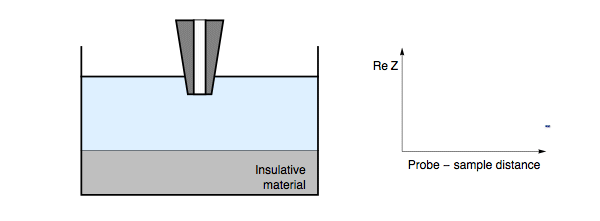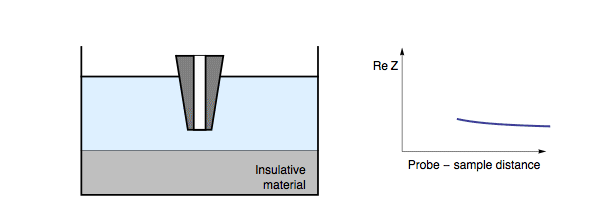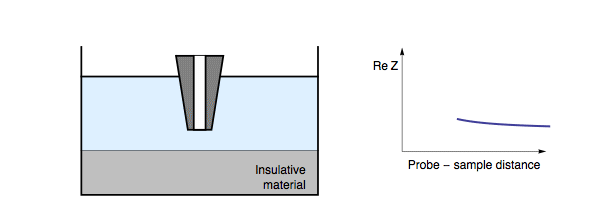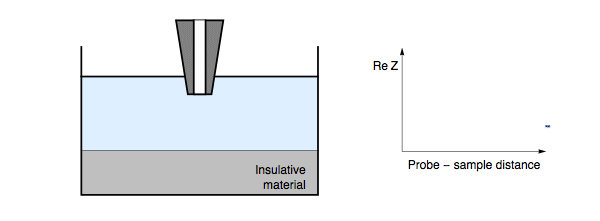
Figure 1: During ac-SECM approach to an insulator as the probe to sample
distance decreases the measured impedance increases. This occurs in all cases
regardless of the measurement frequency and electrolyte.
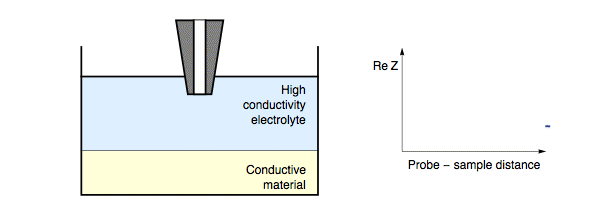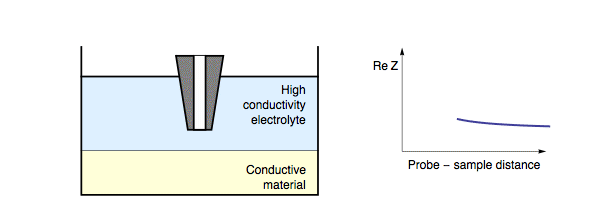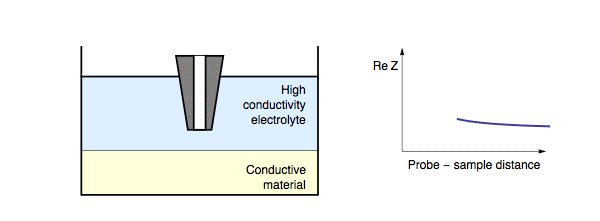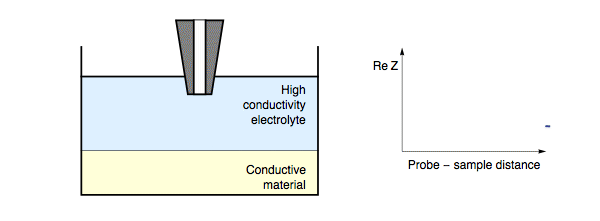
Figure 2: During ac-SECM approach to a conductor in a high conductivity
electrolyte, or with a low measurement frequency, the impedance increases
with decreasing probe to sample distance.
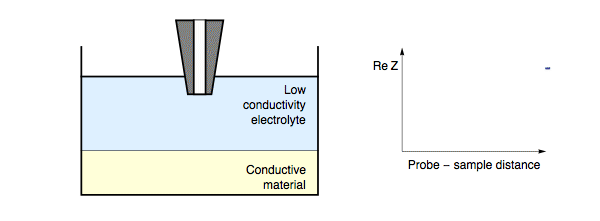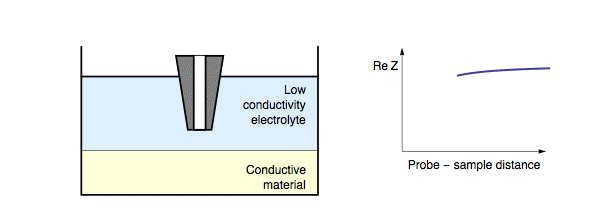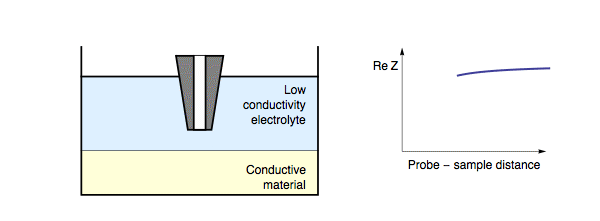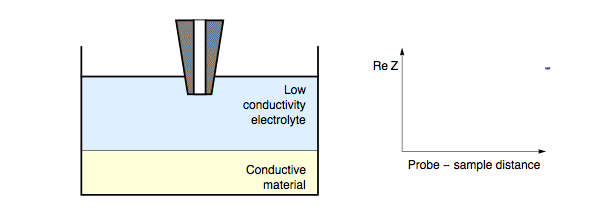
Figure 3: During ac-SECM approach to a conductor in a low conductivity
electrolyte, or with a high measurement frequency, the impedance decreases with
decreasing probe to sample distance.
As with dc-SECM the signal measured in ac-SECM can be seen to relate to both the sample conductivity and its topography (as reflected by changes in the tip-to-sample distance). Therefore, in some cases, particularly for very rough samples, it can be beneficial to measure ac-SECM in constant distance mode to track the sample topography throughout. Intermittent Contact (ic)-SECM can be used to simultaneously track and measure the sample topography throughout the measurement, however discussion of this technique is outside the scope of this article.
Basic Alternating Current-Scanning Electrochemical Microscopy measurement types
As with dc-SECM there are two basic experiment types used in ac-SECM: the approach curve and the area scan.
During an ac-SECM approach curve the probe is moved towards the sample surface with the impedance measured as a function of the probe position in z. Changes in the impedance allow the relative proximity of the probe to the sample to be determined. The ac-SECM approach curve is therefore commonly used to determine the probe position for imaging measurements.
The other basic experiment type is the area scan. In these measurements, the probe is raster scanned across the sample surface, whilst in close proximity to it. The result is a map correlating impedance to probe position, allowing the electrochemical nature of the sample to be visualized.
What are the components of an Alternating Current-Scanning Electrochemical Microscope?
An Alternating Current-Scanning Electrochemical Microscope is composed of a number of components that are annotated in Fig. 4. All ac-SECMs have an x,y,z scanning stage to allow for the 3D scanning required for ac-SECM measurements. To achieve the highest resolution measurements the x,y,z scanning stage is capable of achieving sub-micron step sizes. In order to apply the required ac signal to the probe to perform an ac-SECM measurement, the bipotentiostat of the instrument must be EIS capable. While the ac bias is only applied to the probe, use of a bipotentiostat allows the application of a dc bias to the sample. An electrochemical cell is needed to mount the sample, and required electrolyte, onto the scanning stage. The electrochemical cell has provisions for removing sample tilt. ac-SECM requires the use of an ultramicroelectrode probe which typically acts as the main working electrode in the measurement.

Figure 4: An Alternating Current-Scanning Electrochemical Microscope is annotated to show all of the components.
Why use Alternating Current-Scanning Electrochemical Microscopy?
Bulk EIS has found widespread use studying the electrochemical processes at a sample interface, however, the information obtained is an average of the entire sample. This requires sometimes difficult data interpretation to determine the local effects on the impedance characteristics of a sample. ac-SECM is a complement of bulk EIS which allows the local impedance of a sample to be visualized, without the typical interpretation necessary for bulk EIS to pinpoint local effects. ac-SECM is only one of a small handful of techniques that allows local measurement of the sample impedance, the others being Local Electrochemical Impedance Spectroscopy, and ac-Scanning Droplet Cell. Of these techniques ac-SECM is the only one that does not have a conductivity requirement for the sample. It can be used to measure anything from a fully conducting to a fully insulating sample and anything in between. As a result, ac-SECM is the only one of these techniques which does not require the sample to act as a working electrode in the measurement, and furthermore does not require any electrical connection to the sample at all. The sample merely needs to be mounted in the electrochemical cell for measurement, making ac-SECM highly applicable for samples that are difficult to prepare for other local and bulk electrochemical techniques, such as biological materials.
In the Scanning Electrochemical Microscopy family, ac-SECM has the unique advantage that it is performed without the need for a redox mediator. This is a particular advantage for those samples which interact poorly with many of the traditional mediator materials. For example, in the field of corrosion, a mediator can act to enhance or inhibit the corrosion process, meaning the dc-SECM measurement may not reflect the real-world processes occurring. As another example, in the field of biology, many of the commonly used redox mediators can be toxic to the living cells under study, therefore ruling out their use. By using ac-SECM instead of dc-SECM, therefore, the range of samples for which SECM can be applied is expanded.
What is Scanning Electrochemical Microscopy used for?
Figure 5: The application areas of ac-SECM are shown.
Alternating Current-Scanning Electrochemical Microscopy is applicable to any field for which the local impedance information of a sample is of interest. ac-SECM is of particular interest in applications where the requirement of a redox mediator to perform dc-SECM measurements would be detrimental to the sample under study, for example, some biological and corrosion samples. The fields in which ac-SECM can be applied are shown in Fig. 5. Some examples of the use of ac-SECM include:
- Investigations into hydrogen embrittlement [3]
- Studies of cell morphology [4]
- Analysis of the effect of grains and grain boundaries on the on the conductivity of solid electrolytes for batteries [5]
The uses of ac-SECM, and other scanning probe electrochemical techniques, are covered in more detail in a series of Learning Center articles dedicated to the research questions which can be answered by these techniques. These include a look at the use of scanning probe electrochemistry into batteries, biology, corrosion, coatings, and materials.
Further Information
BioLogic has a growing number of resources to grow your understanding of Alternating Current-Scanning Electrochemical Microscopy. More information about using ac-SECM for a variety of applications can be found by consulting the scanning probe workstations application notes. To learn more about achieving the best images using SECM and ac-SECM please see our two-part tutorial: How to get clear images in SECM.
Glossary
Electrochemical Impedance Spectroscopy (EIS): A bulk electrochemical measurement in which a sinusoidal bias is applied to a sample over a set frequency range to obtain information about the electrochemical processes occurring.
Redox mediator: An electrochemically active compound which transfers electrons in SECM, allowing measurement of Faradaic current.
Scanning probe electrochemistry: Any of the family of scanning probe techniques which measures the local electrochemistry of a sample. This includes SECM, SKP, LEIS, SDC, and SVET.
References
- R. Horrocks, D. Schmidtke, A. Heller, A. J. Bard, Anal. Chem. 65 (1993) 3605-3614
- B. Katemann, A. Schulte, E. J. Calvo, M. Koudelka-Hep, W. Schuhmann, Electrochem. Comm. 4 (2002) 134-138
- C. Lafouresse, M.-L. de Bonfils-Lahovary, L. Laffont, C. Blanc, Electrochem. Comm. 80 (2017) 29-32
- M. Diakowski, Z. Ding, Phys. Chem. Chem. Phys. 9 (2007) 5966–5974
- Takami, Y. Morita, M. Yonemura, Y. Ishikawa, S. Tanaka, M. Mori, T. Fukunaga, E. Matsubara, ACS Appl. Energy Mater. 6 (2018) 2546-2554
Related products