Application of the bipotentiostat to an experiment with a Rotating Ring-Disk Electrode (RRDE bipot) Battery – Application Note 7
Latest updated: November 28, 2023Abstract
In this application note, we demonstrate the capability offered by BioLogic multi-channel (VMP3, VSP, SP-300, etc.) potentiostats/galvanostats to run bipotentiostat experiments with rotating ring-disk electrodes. Configured as a bistat and coupled with an RRDE, the system is a powerful tool for studying electrochemical mechanisms in solution.
Introduction
The aim of this document is to show the capability offered by a multichannel potentiostat (VSP-300, BP-300, VMP-3, VMP-300) to run experiments with a rotating Ring-Disk electrode system.
The SP-300 and BP-300 are two-slots potentiostats/galvanostats especially designed to work as a bipotentiostats. The bipotentiostat is mainly used in electro-chemical experiments with two working electrodes in a well-defined hydrodynamic regime. The hydrodynamic regime is ensured with a rotating electrode driven by a rotating power unit. The working electrode in the electrochemical cell typically consists either of a single disk or of a ring-disk electrode. The rotating ring-disk electrode was developed by Frumkin and Nekrasov in 1959. The theoretical description of the electrochemical kinetics at a rotating ring-disk electrode was definitely achieved in 1971 [1]. The applications of the RRDE are numerous in the fields of electrochemical analysis, especially in reaction mechanism studies. The rotating electrode is often used for the detection of short life intermediate species.
Description and Principle
The ring-disk electrode is composed of two electrically isolated electrode surfaces. The ring and the disk electrodes are made of conducting materials such as platinum, gold, silver, copper or glassy carbon. The material of each electrode can be different and are separated by an isolating material such as PTFE or PEEK.
The electrochemical solution flow near the electrode surface describes a spiral that is growing from the middle to the periphery of the surface. The electroactive species move from the disk to the ring electrode. When used in the ring-disk configuration, the ring and the disk electrodes are usually polarized with different potentials, ER and ED, using both channels of the bipotentiostat. For example ED could be an oxidation potential and ER a reduction potential. The electrochemical reactions that can take place on the electrode surface according to the applied potential can be defined as follow:
$\mathrm{Disk:\ X\ \leftrightarrow\ Y\ +\ }ne^-$
if ED enough positive
$\mathrm{Ring:\ Y\ +\ } ne^-\ \mathrm{\leftrightarrow\ Y\ }$
if ER smaller than ED (enough for the reduction).
The Y compound made on the disk electrode is moved by diffusion and convection to the ring electrode where it can be electro-chemically detected and studied. One possible experiment is to sweep the potential on the disk electrode from a reduction potential to an oxidation potential and to fix a reduction potential on the ring electrode to detect by amperometry the species made on the disk.
For this kind of experiment, the electro-chemical cell also contains a reference electrode and a counter electrode that are common for both working electrodes. The characteristic value that must be considered is the collection efficiency of the ring electrode: N = – IR/ID
This coefficient is characteristic of the RRDE.
Experimental Part
The electrochemical cell contains a four electrode system with two working electrodes (rotating ring-disk electrode), one Pt counter electrode and one silver/silver chloride electrode as reference.
The solution used in the experiment is made with Fe(CN)64- (5.10-3 mol·L-1) with KCl (0.5 mol·L-1) as support electrolyte. The instrument used is a VMP-3 with EC-Lab software version 11.21. The connection used is in the “CE to Ground” mode with one common reference and one common counter electrode.
There are three bipotentiostat techniques presented on the following figure:
![]()
Figure 1: Bipotentiostat techniques (window “Insert Techniques”).
In this experiment, Cyclic Voltammetry & Chronoamperometry technique (CV-CA) has been used. Bipotentiostat techniques require two channels: one for each working electrode, the disk and the ring. We can choose which channel is linked to which electrode by going to “Edit” -> “Group / Synch / Stack / Bipot” (Fig. 2)
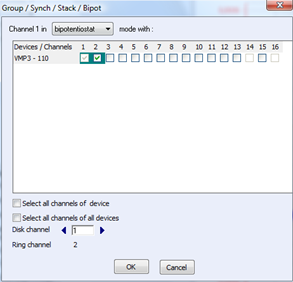
Figure 2: Window “Group / Synch / Stack / Bipot”.
The parameters of the experiment are shown on the following figure (Fig. 3).
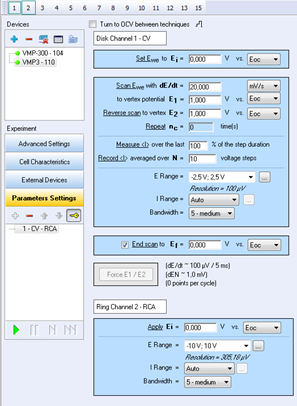
Figure 3: Parameters of the experiment.
Results
The VMP-3 is used as a bipotentiostat. On the disk electrode surface a potentiodynamic mode is applied (2000 rpm) from 0 V/OCV to 1 V/OCV in order to oxidize the Fe(II) species into Fe(III) species. At the same time on the other channel connected to the ring electrode, a potentiostatic mode with 0 V/OCV is applied in order to reduce the Fe(III) species previously formed on the disk electrode. The curves are shown in the following figure.
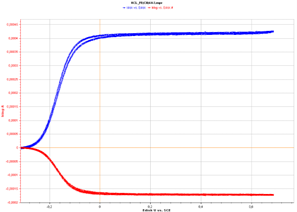
Figure 4: Overlayed chronoamperometric curves obtained simultaneously on the disk (blue) and on the ring (red) electrodes. (I vs. Edisk(V/REF))
Both experiments were synchronized to apply the constant potential on the ring electrode when the potential sweep on the disk electrode started. The curves clearly show that the Fe(III) formed species during the oxidation step are partly reduced on the ring electrode. The quantity of reduced iron is growing with the quantity of Fe(III) formed.
The diffusion limit currents on the disk and on the ring electrodes have been obtained thanks to the previous curves (Fig. 4). We can obtain the collection efficiency:
$N = I_{\mathrm{Ring}}/I_{\text{Disk}} = 0.405$
The calculated collection efficiency according to the previous curves is 40.5 %.
The calculated collection efficiency according to the previous curves is 40.5 %. The theoretical collection efficiency has been calculated using an expression given in [2] and gives a value of 42.5 %, which is a little higher than the calculated one, but similar.
Conclusion
Coupled with a bipotentiostat, the rotating ring-disk electrode is a powerful tool that can be used to effectively study electrochemical mechanisms in solution. The feasibility of linked experiments with rotating electrode control has been clearly demonstrated in this application note. Moreover, in the case of both working electrodes (ring + disk), the experiment has shown a similar collection efficiency as defined by the supplier.
References
- W.J. Albery and M.L. Hitchman, Clarendon press Oxford, (1971).
- W.J. Albery and S. Bruckenstein, Trans. Faraday Soc., 62 (1966) 1920.
Revised in 07/2018