Sensor pulsed techniques: SWV, DPV & NPV Electroanalysis & Electrochemistry Sensor – Application Note 67
Latest updated: May 22, 2024Abstract This note introduces three of the most common pulsed techniques used in electrochemistry, which are available in EC-Lab®. Pulsed techniques are mainly used to detect reactants at very low concentrations. In some conditions, they can be preferred over other voltammetric techniques, such as cyclic voltammetry on a static or a rotating electrode.
Introduction
The pulse voltammetric techniques are electroanalytical techniques mainly used to detect species of very small concentrations. (10-6 to 10-9 mol·L-1). They were developed to improve voltammetric polarography experiments, in particular by minimizing the capacitive (charging) current and maximizing the faradaic current.
The polarography was invented by Prof. Heyrovský (for which he won a Nobel prize) and consists in using a droplet of mercury as an electrode, that grows, falls and is renewed.
The main advantages of using a mercury drop electrode are that i) its surface and the diffusion layer are constantly renewed, and not modified by deposited material during electrochemical processes and ii) the proton reduction on mercury occurs at very high cathodic overpotentials. Thus, it is possible to observe reactions occurring at large potential values.
Nowadays, European regulations (RoHS: Restriction of Hazardous Substances) limits the use of mercury, famous for being a highly dangerous metal, consequently the pulse techniques are used with non-polarographic solid electrodes.
EC-Lab® offers six different pulse techniques.
Among them, Normal Pulse Voltammetry (NPV) and Differential Pulse Voltammetry (DPV) were developed along with polarography: the potential increase of the step corresponds to the growth of mercury drop while the potential decrease of the step corresponds to the drop fall. Other pulse techniques such as Reverse Pulse Voltammetry (RPV) and Square Wave Voltammetry (SWV) were developed outside of the polarographic context [1].
In this note, the analytical characteristics of the classical (Cyclic Voltammetry on a static electrode (CV) and Cyclic Voltammetry on a Rotating Disk Electrode (RDE)) and pulsed (NPV, DPV, SWV) voltammetric techniques are compared.
THEORETICAL DESCRIPTION
At low concentration, the measured current is mainly constituted by capacitive current. The intrinsic characteristics of the pulsed techniques allow the user to improve the detection process, for example the detection limit (DL) can reach 10 nmol·L-1. Indeed, the faradaic current, IF Eq. (1), decreases more slowly than the capacitive current, IC Eq. (2), the subtraction (Fig. 1) of the current just before and after the potential pulse (some mV during some ms) gives mainly the faradaic current.
The faradaic current is given by the following equation [2]:
$$I_{\mathrm{F}} = nFAC\sqrt{\frac{D}{\pi t}}\tag{1}$$
With n the number of electrons involved in the redox process, F the Faraday constant, A the surface of the electrode, C the concentration of the electroactive species, D the diffusion coefficient of the electroactive species, t the time after the application of the pulse at which the current is sampled.
The capacitive current is given by the following equation [2]:
$$I_{c} = \frac{E}{R}\mathrm{exp}\left(\frac{-t}{RC_{\mathrm{dl}}}\right)\tag{2}$$
With E the pulse potential, R the ohmic resistance between the working electrode and the reference electrode, Cdl the double layer capacitance.
Figure 1 shows the sequences of each pulsed voltammetric technique.
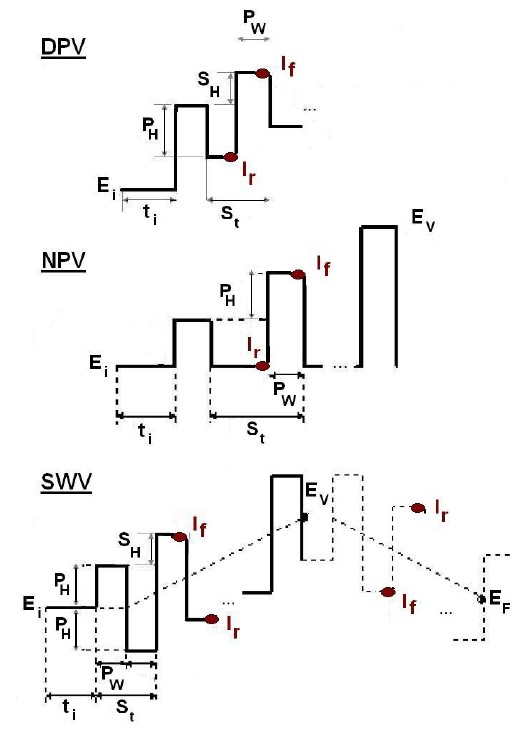 Figure 1: Measurement sequences of the pulsed techniques.
Figure 1: Measurement sequences of the pulsed techniques.
Table I summarizes the current expression for each voltammetric technique. Each relationship can be used to determine the concentration of the species of interest. Ip is the peak current (= maximum current) and Il the mass-transport limited current [3]. Where ν is cinematic viscosity of the electrolyte in cm²·s-1, and ω the rotation rate of the electrode in rpm.
Table I: Current expressions for each voltammetric technique.
| Techniques | |
|---|---|
| CV | $$I_\text{p} = -0.446n^{3/2}A\sqrt{D\upsilon}C$$ |
| RDE | $$I_\text{l} = 0.620nFACD^{2/3}\upsilon^{-1/6}\omega^{1/2}$$ |
| NPV | $$I_\text{p} = nFAC\sqrt{\frac{D}{\pi t}}$$ |
| DPV | $$I_\text{p} = nFAC\sqrt{\frac{D}{\pi t}}\left(-\text{tanh}\left(\frac{nFE}{4RT}\right)\right)$$ |
| SWV | $$I_\text{p} = 2.67nFAC\sqrt{\frac{D}{\pi t}}$$ |
1 NPV (NORMAL PULSE VOLTAMMETRY)
The oldest technique is the Normal Pulse Voltammetry. It directly emanates from polarography. It consists in applying a potential Ei to the sample for which the reaction of interest does not occur and apply a series of successive potential steps of PW lengths and whose amplitudes is a multiple of PH (Fig. 1). The current is measured over the potential step and should be preferably sampled at the end of the potential step (If), where the charging (capacitive) current is minimized and the faradaic current maximized. Typical values for these parameters are [4]:
St = 1 s; PW = 50 ms; PH/St = 2 mV/s.
2 DPV (DIFFERENTIAL PULSE VOLTAMMETRY)
Also directly coming from polarography, DPV allows to obtain higher sensitivities than NPV. In DPV, the base potential is not constant but is changed steadily in small increments (SH). The pulse height, PH, is only 10 to 100 mV and is maintained at a constant level with respect to the base potential (Fig. 1). Two current samples are taken: Ir immediately before the pulse and If late in the pulse and just before the potential decrease. The record of the experiment is a plot of the current difference, δI = If – Ir versus the base potential [1]. Typical values for the parameters are [4]:
St = 1 s; SH+PH = 50 mV; PW = 50 ms; SH/St = 2 mV/s
3 SWV (SQUARE WAVE VOLTAMMETRY)
This technique was pioneered by Osteryoung [3] and is the first one that does not come from polarography (although Barker used what he called Square Wave Polarography [5]) as it is only possible due to the advent of microprocessor-controlled potentiostats/galvanostats. The square wave voltammetric waveform combines a large-amplitude square wave modulation with a staircase wave-form (Fig. 1). Again, two current samples are taken: If late in the pulse and just before the potential decrease and Ir immediately before the pulse. Typical values for the parameters are [4]:
St = 5 ms; PH = 25 mV; SH = 10 mV.
Please note that in this document If – Ir will be referred to as I delta.
EXPERIMENTAL CONDITIONS
The investigations are carried out using a BioLogic VMP3 potentiostat/galvanostat with standard channels and EC-Lab® software. The solution was composed of K4Fe(CN)6 in a concentration range between 1.1 mmol·L-1 and 1.1 μmol·L-1 in water with KCl (0.1 mol·L-1) as a supporting salt.
The equation of the reaction is the following:
$$[\mathrm{Fe(CN)_6]^{-4}} \longleftrightarrow [\mathrm{Fe(CN)_6]^{3-}} + e^- \tag{3}$$
A three-electrode set-up is used: a platinum electrode with a surface A = 0.196 cm2 as a working electrode, an Ag/AgCl reference electrode, a Pt wire as a counter electrode. The parameters used for the cyclic voltammetry and RDE techniques are shown in Fig. 2. Please note that the rotation of the RDE was 500 RPM.
Please note that for RDE experiments the voltage scan was only performed in the forward direction.
The parameters used for the pulsed techniques are shown in Fig. 3. The window shows parameters specific to DPV technique but the same parameters were used for NPV and SWV.
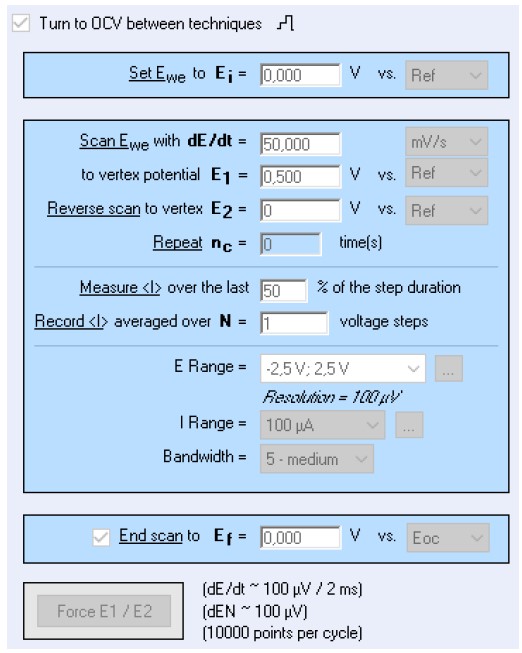
Figure 2: EC-Lab® parameters for the CV and RDE experiments.
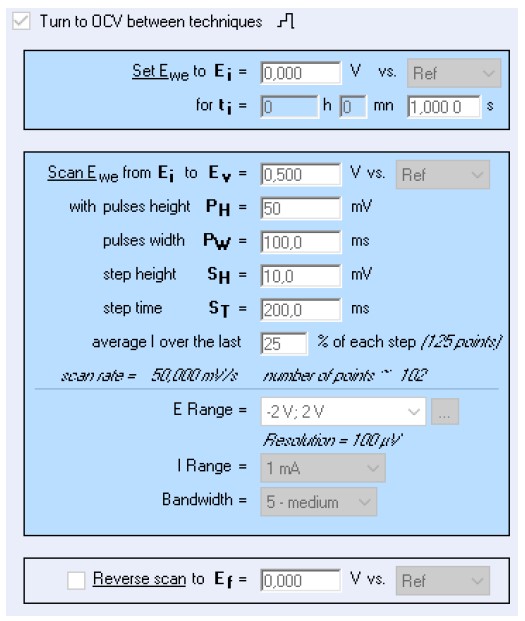
Figure 3: EC-Lab® parameters for the DPV experiment. The same parameters were used for NPV and SWV.
RESULTS AND DISCUSSION
Figure 4 shows the I vs. Ewe curves obtained using CV on a quiescent electrode for different concentrations of ferrocyanide. 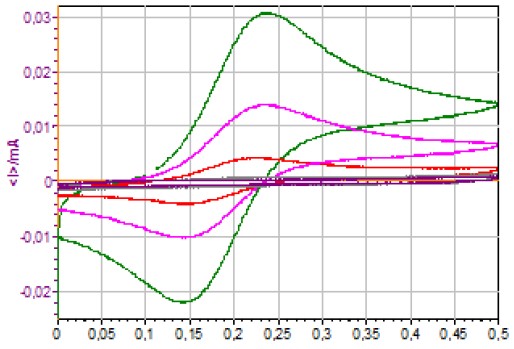
Figure 4: I vs. Ewe curves obtained using CV on a quiescent electrode for different [Fe(CN)6]4-concentrations (1127, 502, 180, 11.2 and 1.1 μmol·L-1).
Figure 5 shows the I vs. Ewe curves obtained using CV on an RDE for different concentrations of ferrocyanide. 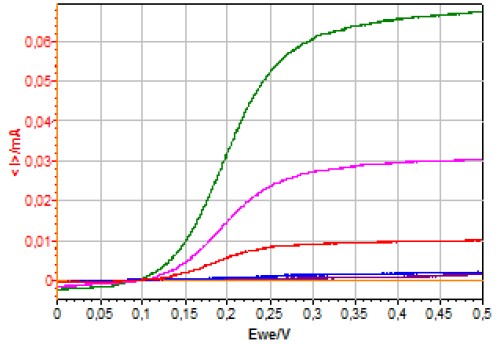 Figure 5: I vs. Ewe curves obtained using CV on a RDE at 500 RPM for different [Fe(CN)6]4- concentrations (1127, 502, 180, 11.2 and 1.1 μmol·L-1).
Figure 5: I vs. Ewe curves obtained using CV on a RDE at 500 RPM for different [Fe(CN)6]4- concentrations (1127, 502, 180, 11.2 and 1.1 μmol·L-1).
Figure 6 shows the I delta vs. E step curves obtained using DPV on a static electrode for different concentrations of ferrocyanide. Please note that E step is equivalent to Ewe and is the value resulting from the potential sweep.
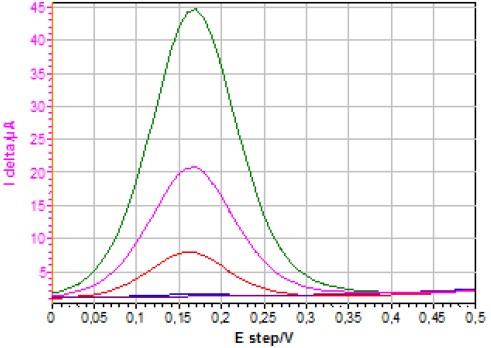
Figure 6: I delta vs. E step curves obtained using DPV on a quiescent electrode for different [Fe(CN)6]4- concentrations (1127, 502, 180, 11.2 and 1.1 μmol·L-1).
Figure 7 shows the I delta vs. E step curves obtained using SWV on a quiescent electrode for different concentrations of ferrocyanide. 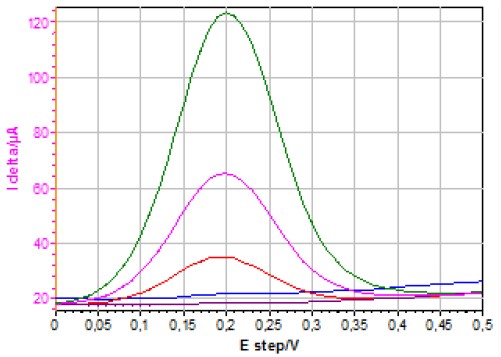
Figure 7: I delta vs. E step curves obtained using SWV (PH = 50 mV, PW = 100 ms, SH = 10 mV) on a quiescent electrode for different [Fe(CN)6]4- concentrations (1127, 502, 180, 11.2 and 1.1 μmol·L-1).
Figure 8 shows the I delta vs. E step curves obtained using NPV on a quiescent electrode for different concentrations of ferrocyanide.
Anodic currents are measured with the “Peak Analysis” (for CV, DPV and SWV) or “Wave Analysis” (for RDE and NPV) tools in the “Analysis” menu of EC-Lab® software.
The sensitivity, given by the slope of the Ip or Il vs. Fe concentration plot (Fig. 9 and Tab. II), is mostly larger for pulsed methods in the conditions used in the present paper.
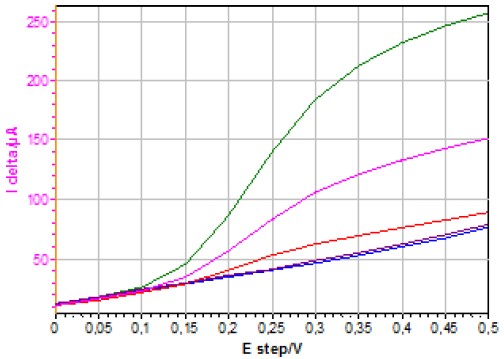
Figure 8: I delta vs. E step curves obtained using NPV (PH = 50 mV, PW = 100 ms, ST = 200 ms) on a quiescent electrode for different [Fe(CN)6]4- concentrations (1127, 502, 180, 11.2 and 1.1 μmol·L-1).
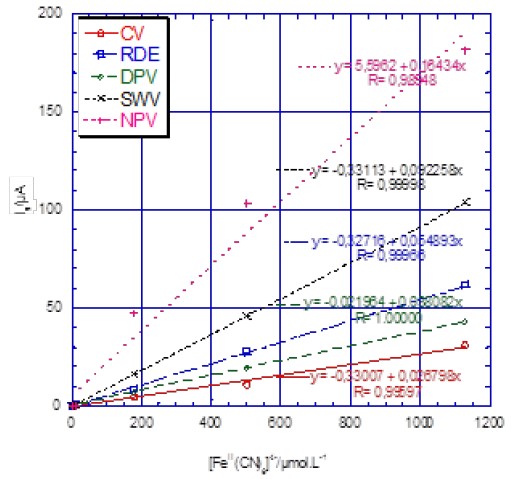
Figure 9: Plot of the peak current Ip (CV, DPV, SWV) and limited current Il (RDE and NPV) vs. [Fe(CN)6]4- concentration.
Voltammetry on an RDE, with constant matter transport, is less sensitive than SWV and NPV. The SWV is four and two times more sensitive than DPV and NPV, respectively. This technique is also faster than the other methods. Moreover, because of the DPV and SWV peak-shaped response, the standard redox potential resolution is sharper.
Consequently, two species with close oxidation or reduction potentials (down to ΔE = 50 mV) can be discriminated. This is an additional advantage for pulsed methods. They are not only more sensitive but also more selective.
Table II: Sensitivity of voltammetric techniques.
| Technique | Sensitivity/µA L mmol-1 |
|---|---|
| CV | 27 |
| RDE | 38 |
| DPV | 55 |
| SWV | 92 |
| NPV | 164 |
In addition, thanks to the peak-shaped signal, the Detection Limit (DL) can be decreased. For DPV measurement, the DL is 1 μmol·L-1 whereas for CV, it is around 10 μmol·L-1 with this non-optimized procedure. Finally, the DL can also be improved by using the low current board available for both VMP3 and VMP-300 technologies.
Conclusion
In this note we have introduced three of the six pulsed techniques available in EC-Lab® and compared them with Cyclic Voltammetry on a static and rotating disk electrode in terms of sensitivity and detection limit. In the experimental conditions of this paper, the best results were obtained with NPV and SWV techniques. It is generally considered that pulsed techniques can be optimized to provide the best sensitivity.
References
1) A.J. Bard, L. R. Faulkner in : Electrochemical Methods, Fundamentals and Applications, Wiley and Sons, 2nd ed. (2001) Chapter 7.
2) Analytical Electrochemistry, J. Wang ed. Wiley and Sons, (2000) Chapter 3.
3) Application Note#56 “Electrochemical reaction kinetics measurement: the Levich and Koutecký-Levich analysis tools”
4) J. G. and R. A. Osteryoung, Anal. Chem. 57 1 (1985) 101 A
5) G. C. Barker, A. W. Gardner, Fresenius Zeitschrift für Analytische Chemie, 173, 1 (1960) 79