SECM without the S
Latest updated: November 3, 2025Introduction
When Scanning Electrochemical Microscopy (SECM) comes to mind it is typically the heat map images of current made by raster scanning a probe in close proximity to the sample of interest, however, this is not the only way to use SECM. Even without raster scanning SECM can be a powerful technique, offering the ability to quantify adsorbed species, study ion fluxes, and investigate the effect of the substrate on species formation amongst other uses. This article will focus on examples of SECM performed at a single x,y,z point, so while approach curves will not be addressed, their applications beyond probe positioning can be explored in the article: How to use SECM approach curves for energy applications.
Single point measurements
The majority of single point measurements performed in SECM are in the dc-mode, as such the bulk of this article will focus on these experiment types, with a brief focus on ac-SECM for single point measurements.
Surface Interrogation (SI)- SECM
Surface Interrogation (SI)-SECM is becoming more interesting with the growing popularity of SECM to study electrocatalytic systems. SI-SECM is a dc-SECM technique which is used for quantification of species adsorbed at a substrate. While it can be used in a range of fields its most popular use is in studies of catalysts. SI-SECM requires a four-electrode cell, with both the probe and the sample acting as working electrodes, requiring a bipotentiostat setup. The sample in SI-SECM is typically made to be the same diameter as the SECM probe, however use of macroelectrode substrates for SI-SECM has been demonstrated [1].
To perform an SI-SECM experiment the probe is first approached to the sample surface, so it has a normalized distance, $L$, of ≤ 0.3. The normalized distance is defined by:
$$L = d/a $$
Where $d$ is the distance between the probe and sample and $a$ is the probe radius [2]. Following approach both probe and sample are returned to Open Circuit Potential (OCP). With the probe in close proximity to the sample surface, the sample is biased to produce an intermediate or product adsorbed on its surface. Once the adsorbate has formed the sample is returned to OCP. Cyclic Voltammetry (CV), Linear Sweep Voltammetry (LSV), or ChronoAmperometry (CA) is then performed at the probe, allowing it to act as a titrant generator and collector. During the experiment at the probe the signal measured is dependent on the presence of adsorbate. While the adsorbate is present positive feedback occurs, once the adsorbate is fully consumed negative feedback occurs. These steps are outlined in Fig. 1.
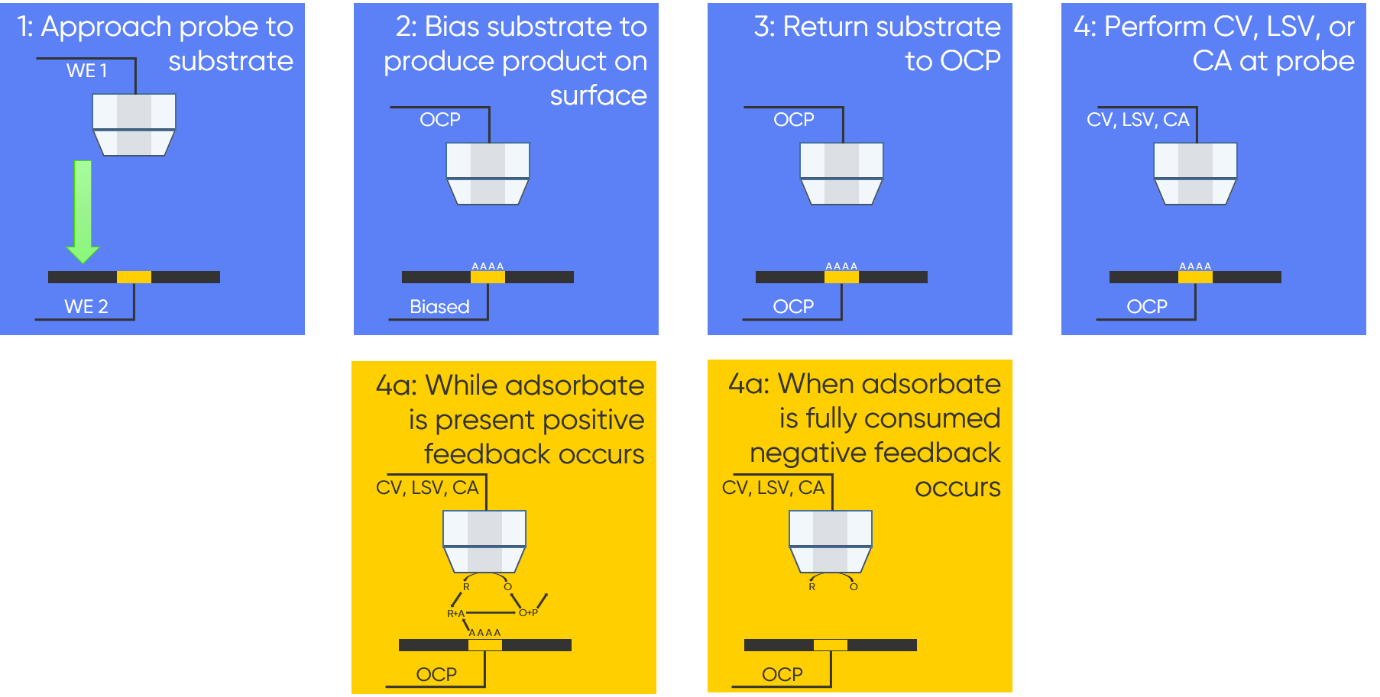
Figure 1 : The steps involved in an SI-SECM experiment are outlined.
Tip Substrate Voltammetry (TSV)-SECM
Closely related is the Tip Substrate Voltammetry (TSV)-SECM, which provides direct information about ion fluxes in solution related to adsorption, desorption, and dissolution of species. It is particularly useful for catalysis, and materials studies. In this mode the probe is again approached to the substrate, which is the second working electrode in the electrochemical cell. When in close proximity to the sample, the probe is biased at a set potential, a CV or CA is then run at the sample. Because of the close proximity between the probe and sample any electrochemically active species produced at the substrate are detected by the biased probe. The steps used to perform TSV-SECM are outlined in Fig. 2.
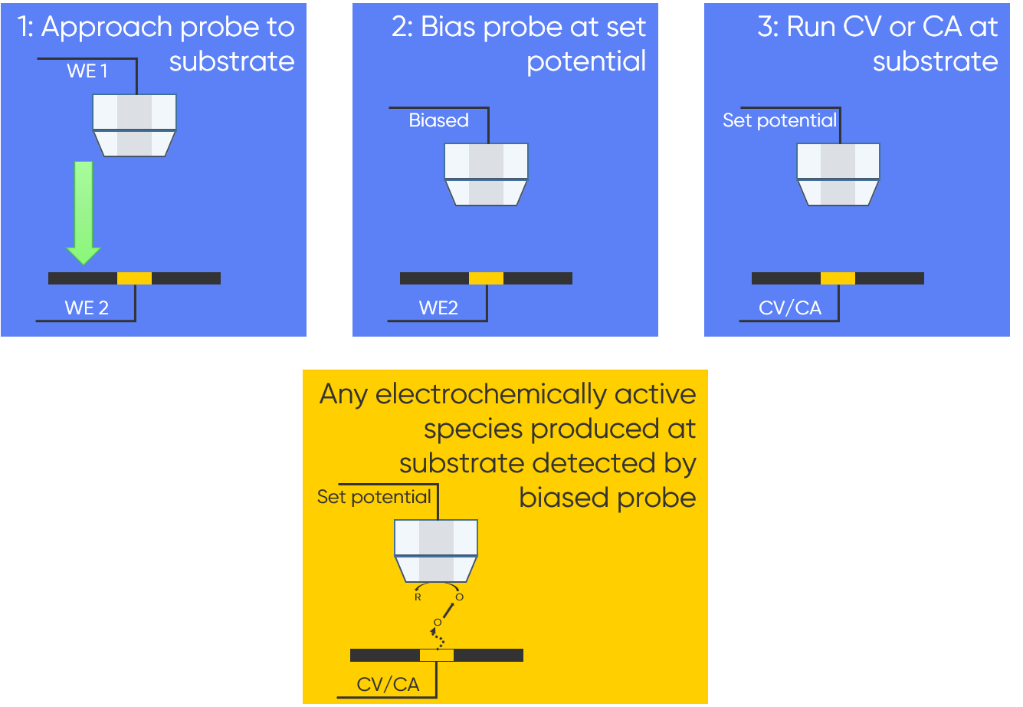
Figure 2 : The steps involved in a TSV-SECM experiment are outlined.
Single point Substrate Generation/Tip Collection (SG/TC)-SECM
The opposite can also be performed, and is considered as an extension of the Sample Generation / Tip Collection (SG/TC) mode. It is used to investigate the effect of substrate potential on formation of electroactive species [3]. In this version the probe is in close proximity to the sample surface which is biased as the second working electrode. A CV or CA can then be run at the probe. The bias applied to the sample can be changed between electrochemical experiments at the probe, to investigate its effect. The steps involved in this single point SG/TC mode are outlined in Fig. 3.
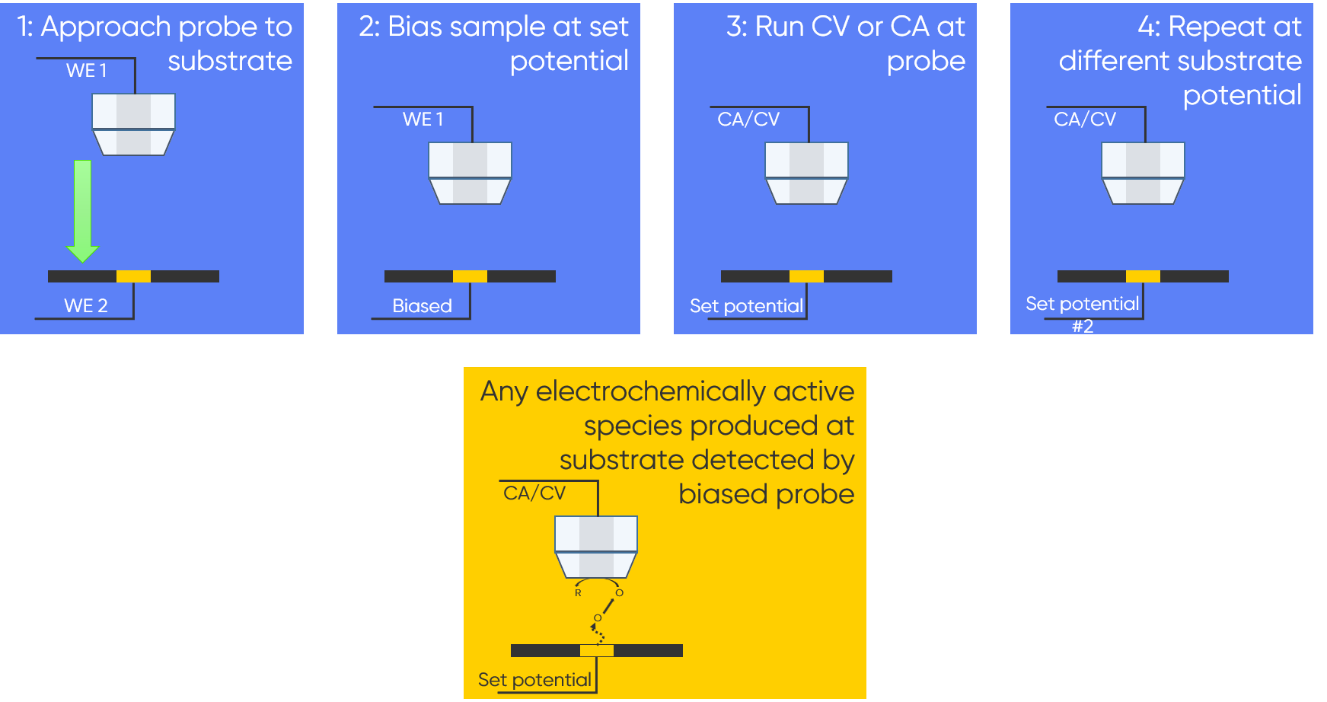
Figure 3 : The steps involved in a single point SG/TC-SECM experiment are outlined.
ac-SECM
Briefly looking at ac-SECM, this can also be used to perform single point measurements, in this case Local Electrochemical Impedance Spectroscopy (LEIS) can be performed. Bulk EIS is an average measure of the sample of interest, in heterogeneous samples this may not provide the full picture of the sample, in which case local EIS can be used. In these experiments because the SECM probe is in close proximity to the sample surface the EIS experiment performed at the probe reflects the nature of the sample [4-5].
Conclusion
SECM is well known for its ability to image the electrochemical nature of a sample surface. As outlined, single point measurements can also be a great use of SECM. In particular single point SECM measurements, like SI-SECM and TSV-SECM, are of interest for catalysis studies.
References
- Papaderakis, D. Tsiplakides, S. Balomenou, S. Sotiropoulos, Electrochem. Comm. 83 (2017) 77-80
- Yang, L. Yang, W. Yong, P. Li, M. Zhou, Z. Jin, Chem. Sci. (2025) Advance Article
- Pandiarajan, S. Ravichandran, J. Electrochem. Soc. 169 (2022) 106505
- Lucas, J.-F. Boily, Langmuir 31 (2015) 13618–13624
- Lucas, J.-F. Boily, J. Phys. Chem. C 121(2017) 27976−27982





